Scheme 1. Synthesis of the bicyclo[3.3.1]nonane core in N-desmethyl-α-obscurine through formal (3+3)-cycloadditiona.
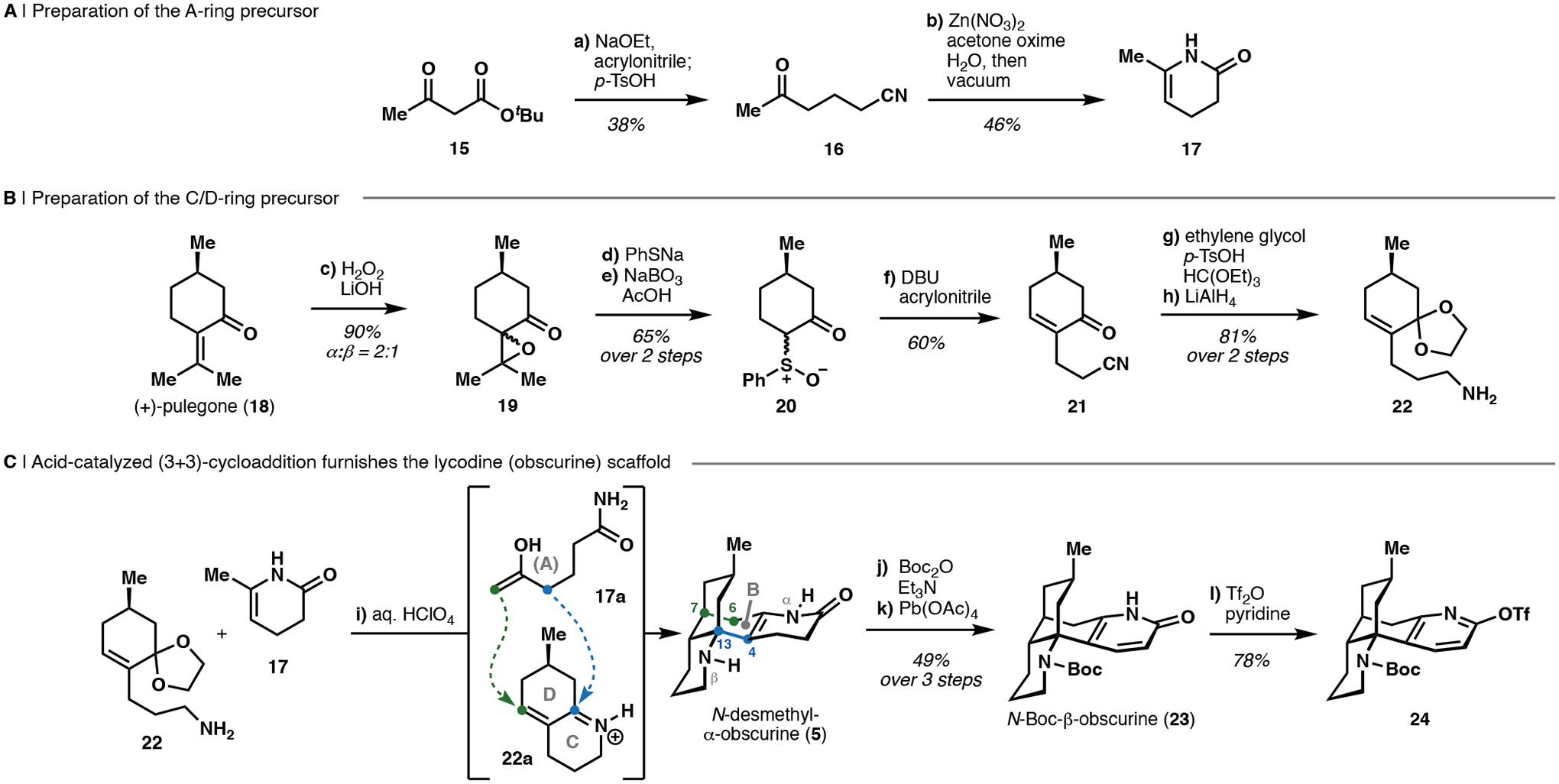
aReagents and conditions: (a) NaOEt, EtOH, 21 °C, then acrylonitrile, 0 to 21 °C, then TsOH, 145 °C (38%, >13 g scale); (b) Zn(NO3)2 • 6 H2O, acetone oxime, H2O, 90 °C, then vacuum, 120 °C (46%, >2 g scale); (c) aq. H2O2, LiOH• H2O, MeOH, H2O, 21 °C (90%, 30 g scale); (d) PhSH, Na, THF, 21 °C, then 19, 85 °C; (e) NaBO3• H2O, AcOH, 40 °C (65%, 2 steps, >17 g scale); (f) DBU, iPrOH, 0 °C, then acrylonitrile, 0 to 40 °C (60%, >7 g scale); (g) ethylene glycol, p-TsOH, HC(OEt)3, 75 °C (97%); (h) LiAlH4, Et2O, 0 °C (84%, >2 g scale); (i) aq. HClO4, 1,4-dioxane, 105 °C; (j) Boc2O, Et3N, THF, 60 °C (54%, 2 steps); (k) Pb(OAc)4, CHCl3, 21 °C (90%); (l) Tf2O, pyridine, CH2Cl2, −78 to 21 °C (78%).
