Abstract
Background:
Childhood cancer survivors experience significantly higher rates of hypertension which potentiates cardiovascular disease, but the contribution and relationship of genetic and treatment factors to hypertension risk are unknown.
Objectives:
To determine the contribution of a blood pressure polygenic risk score (PRS) from the general population and its interplay with cancer therapies to hypertension in childhood cancer survivors.
Methods:
Using 895 established blood pressure loci from the general population, we calculated a PRS for 3572 childhood cancer survivors of European ancestry from Childhood Cancer Survivor Study (CCSS) original cohort, 1889 from CCSS expansion cohort, and 2534 from the St. Jude Lifetime Cohort (SJLIFE). Hypertension was assessed using National Cancer Institute criteria based on self-report of a physician diagnosis in CCSS and by blood pressure measurement in SJLIFE.
Results:
In the combined sample of 7995 survivors, those in the top decile of the PRS had an odds ratio (OR) of 2.66 (95% CI=2.03–3.48) for hypertension compared to survivors in the bottom decile. The PRS-hypertension association was modified by being overweight/obese (per SD interaction OR=1.13; 95% CI=1.01–1.27) and exposure to hypothalamic-pituitary axis radiation (per SD interaction OR=1.18; 95% CI=1.05–1.33). Attributable fractions for hypertension to the PRS and cancer therapies were 21.0% and 15.7%, respectively, they jointly accounted for 40.2% of hypertension among survivors.
Conclusions:
A blood pressure PRS from the general population is significantly associated with hypertension among childhood cancer survivors and contributes to approximately one quarter of hypertension risk among survivors. These findings highlight the importance of screening for hypertension in all childhood cancer survivors, and identify higher risk subgroups.
Keywords: Hypertension, childhood cancer survivors, polygenic risk score, cancer therapies
Introduction
With improvements in survival rates of childhood cancer over the past decades, cardiovascular disease has emerged as a leading cause of non-cancer morbidity and mortality in long-term survivors(1–5). Compared to siblings, survivors are at five-fold increased risk of developing severe cardiac events(3) and at eight-fold increased risk of cardiovascular disease-related death(6). While much of this risk can be attributed to exposure to cardiotoxic therapies including chest-directed radiation and/or anthracyclines, aging long-term survivors also develop traditional, modifiable risk factors that are primary contributors of cardiovascular disease in the general population, including hypertension, diabetes, dyslipidemia and obesity(7). In survivors, hypertension was independently associated with up to a 19-fold increased risk of cardiovascular diseases and the combined effect of anthracycline and hypertension was significantly greater than the expected additive effects with an 86-fold increased risk of heart failure(7). These results suggest that hypertension can potentiate cancer treatment-related cardiotoxicity in a near-multiplicative manner(8,9).
The prevalence of hypertension in childhood cancer survivors increases sharply with age, exceeding 70% by age 50 and is substantially higher than the general population after accounting for age, sex, race/ethnicity, and body mass index(10). The Children’s Oncology Group (COG) long-term follow-up guidelines for survivors of childhood cancer currently designate chemotherapies (ifosfamide and heavy metals [cisplatin, carboplatin and oxaliplatin]), abdominal, hypothalamic-pituitary axis (HPA) or total body radiation and nephrectomy as risk factors for hypertension, and recommend active surveillance of blood pressure in exposed survivors(11). However, hypertension could be attributed to these cancer treatments in only 9.3% of hypertensive survivors(12) and no other models of risk prediction for hypertension are currently available. As such, there is a need to enhance risk stratification in order to identify survivors at increased risk of hypertension who can be targeted for enhanced surveillance and lifestyle and pharmacological interventions that may minimize cardiovascular burden.
In the general population, both heritable and lifestyle risk factors contribute to the risk of hypertension. To date, 901 genetic variants are known to affect blood pressure levels in European populations and their cumulative effect, assessed in the form of a polygenic risk score (PRS) (13). This PRS is associated with a 3.34-fold increased risk of hypertension among individuals at the top decile of the PRS, compared to those at the bottom decile. The objective of this study was to evaluate this blood pressure PRS and its interplay with cancer therapies and being overweight/obese in association with hypertension, using two cohorts of childhood cancer survivors of European ancestry.
Methods
Study Population
Participants were from two large cohort studies of childhood cancer survivors in North America, the Childhood Cancer Survivor Study (CCSS)(14,15) and the St. Jude Lifetime Cohort (SJLIFE)(16). The CCSS is a retrospective cohort study with a longitudinal follow-up of survivors of childhood cancer treated at 31 institutions in the United States and Canada. Study eligibility includes a diagnosis of cancer before age 21 years, and survival at 5 years after diagnosis of childhood cancer. Initial recruitment included survivors diagnosed between 1970 and 1986 (“CCSS original cohort”), which was subsequently expanded to include additional survivors diagnosed between 1987 and 1999 (“CCSS expansion cohort”). The CCSS study methodology and characteristics have been previously described(15).
The SJLIFE(16), initiated in 2007, is a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of 5-year survivors of childhood cancer treated at St. Jude Children’s Research Hospital (SJCRH) since 1962. The Institutional Review Boards at SJCRH and each of the centers participating in the CCSS approved the study, and participants provided informed consent.
Considering the existing blood pressure PRS was derived from individuals of European ancestry via genome-wide association study, our analyses were restricted to survivors of European ancestry. In both CCSS and SJLIFE studies, genetic ancestry was determined by principal component analyses using the 1000 Genomes Europeans as the reference population.
Genetic data
Genotype data in the CCSS original cohort were obtained on Illumina HumanOmni5Exome array. Following the quality control (QC) as described previously(17,18), genotypes were imputed up to the Haplotype Reference Consortium r1.1 (2016) haplotypes using Minimac3(19) implemented in the Michigan Imputation Server. Paired-end whole genome sequencing (WGS) using the Illumina HiSeq X10 and/or NovaSeq sequencers was used to obtain genotype data in the CCSS expansion cohort and the SJLIFE. Details of the WGS, data processing and QC metrics are provided elsewhere(20–22). Principal components were generated based on the genotype data of an independent set of common variants using EIGENSTRAT in PLINK version 1.9(23) and used to control for population stratification.
Phenotypes
Participants in the CCSS completed a multi-item questionnaire at baseline and follow-up evaluations that included age at onset of hypertension based on self-report of medical diagnosis and pharmacologic treatment. In SJLIFE, hypertension was ascertained through measured blood pressure and extent of lifestyle/medical intervention. Using modifications of the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE, version 4.03)(24), hypertension was graded and categorized as mild (grade 1), moderate (grade 2), severe (grade 3), life-threatening or disabling (grade 4), or fatal (grade 5). Survivors in both CCSS and SJLIFE are continuously assessed for hypertension based on survey and clinical ascertainment, respectively. In this study, survivors with CTCAE grade ≥2 at any of the hypertension assessments were considered hypertensive and those with grade <2 in all hypertension assessments were classified as hypertension-free.
Polygenic risk score
The largest genome-wide association study on blood pressure to date including over one million individuals of European ancestry robustly implicated 901 loci(13). Using the reported beta estimates (on blood pressure) of these loci as their weights, a PRS was constructed as a weighted sum of the number of risk alleles carried by an individual. Of the 901 loci, five were identified in non-European populations and are monomorphic in Europeans, and one locus was absent in the CCSS original cohort, resulting in 895 loci for our PRS calculation. Risk alleles and weights of these loci are provided in Supplementary Table 1. The PRS was evaluated as a continuous variable or a categorical variable (1st decile, 2nd-5th deciles, 6th-8th deciles, 9th decile, and 10th decile, where the decile cutoffs were determined by the PRS distribution in the CCSS original cohort, the largest of the three cohorts).
Statistical analyses
For each PRS category, age-standardized prevalence ratio of hypertension was calculated as the ratio of the observed number of hypertensive survivors in each individual cohort to the expected number, which was calculated by applying the age-specific (10-year groups) prevalence of hypertension in the CCSS original cohort, the largest among the three cohorts, to the individual cohorts. The nonparametric bootstrap with 1000 replicates was used to estimate the 95% confidence interval (CI).
Continuous variables are reported with their medians with interquartile ranges (IQRs) and categorical variables are presented with percentages. Multivariable analyses assessing association between the PRS and risk of hypertension were performed in individual cohorts as well as in the combined sample of all survivors from the three cohorts. Association results were presented with estimated odds ratio (OR) and corresponding 95% CIs. The nongenetic baseline model was first fit among all survivors in the three cohorts with logistic regression for hypertension adjusting for the top ten principal components and covariates, where the covariates included age at childhood cancer diagnosis, age at last follow-up, sex, body mass index, smoking status (current or former vs. never) and physical activity (vigorous intensity exercise in metabolic equivalents), cohort (CCSS original cohort, CCSS expansion cohort and SJLIFE), and the six COG risk factors (yes/no) including ifosfamide, heavy metals, abdominal radiation, HPA radiation, total body radiation and nephrectomy. Of the six COG risk factors, multivariable analyses showed ifosfamide was not significantly associated with risk of hypertension (adjusted OR [aOR]=1.18; P=0.328), while a strong and statistically significant association was observed when all alkylating agents (bendamustine [treanda], busulfan, carmustine, chlorambucil [leukeran], cyclophosphamide, ifosfamide, lomustine, mechlorethamine [nitrogen mustard], melphalan, procarbazine, and thiotepa) were considered (aOR=1.59; P<0.001). Therefore, we replaced ifosfamide with alkylating agents (yes/no) in all the analyses. The PRS was then added in two ways: one as a continuous variable (z-score normalized across all survivors) assessing adjusted prevalence of hypertension per standard deviation (SD) change in the PRS; and the other as a categorical variable. Stratified analyses based on body mass index (<18.5 kg/m2 [underweight], 18.5–24.9 kg/m2 [normal weight] or ≥25 kg/m2 [overweight/obese]) and each of the six COG risk factors noted above except total body radiation due to insufficient numbers, were conducted, followed by analyses of their multiplicative interaction with the PRS.
In the combined sample, the attributable fraction (AF) for hypertension was calculated using multivariable logistic regression, adjusting for the same covariates of the regression model above and the top ten principal components. Specifically, we first calculated the predicted prevalence of hypertension (i) in presence of both cancer therapies (alkylating agents, heavy metals, abdominal radiation, HPA radiation, total body radiation and nephrectomy) and the PRS (p11); (ii) in absence of PRS by moving everyone to the bottom decile (the first decile) without changing the cancer therapies (p10); (iii) in absence of the six cancer therapies without changing the PRS (p01); and (iv) with elimination of both cancer therapies and the PRS (p00). The AF of hypertension due to cancer therapies and that due to the PRS were then calculated as (p11−p10)/p11 and (p11−p01)/p11, respectively. The AF of hypertension due to the joint effect of cancer therapies and the PRS, exceeding the individual effects of the two estimated separately above(25), was estimated as (p11−p10−p01+p00)/p11. The following stratified analyses were also conducted: (i) body mass index (<18.5 kg/m2 [underweight], 18.5–24.9 kg/m2 [normal weight] or ≥25 kg/m2 [overweight/obese]; (ii) age (<35 years/≥35 years) and (iii) the COG risk factor(s) showing significant interaction with the PRS. Statistical analyses were performed using R 3.4.0 and two-sided P values <0.05 were considered statistically significant.
Results
After excluding survivors with missing phenotype and/or covariate data and of non-European ancestry, there were 7995 survivors in both the CCSS and SJLIFE for the final analysis. Clinical, demographic and treatment characteristics of all 7995study participants according to cohort are provided in Table 1. Participants in the CCSS original cohort were the oldest with median age at last contact of 41.5 years (IQR 12.4 years), followed by those in the SJLIFE with median age at last contact of 35.5 years (IQR 15.3 years) and in the CCSS expansion cohort with median age at last contact of 30.1 years (IQR 8.5 years). The median body mass index in the CCSS original cohort, CCSS expansion cohort and SJLIFE was 22.8 kg/m2 (IQR 5.5 kg/m2), 24.8 kg/m2 (IQR 6.9 kg/m2) and 26.4 kg/m2 (IQR 8.9 kg/m2), respectively. Of the 7995 survivors, 1678 were hypertensive. The prevalence of hypertension was the highest in the clinically-assessed SJLIFE cohort (28.7%), followed by the CCSS original cohort (22.1%) and expansion cohort (8.5%). The PRS distribution in the three cohorts was similar and followed approximately a normal distribution (Supplementary Figure 1).
Table 1 |.
Demographic and treatment characteristics of eligible participants in this study
| CCSS original cohort (n=3572) |
CCSS expansion cohort (n=1889) |
SJLIFE (n=2534) |
|
|---|---|---|---|
| Characteristics | N (%) | N (%) | N (%) |
| Sex | |||
| Female | 1876 (52.5%) | 994 (52.6%) | 1198 (47.3%) |
| Male | 1696 (47.5%) | 895 (47.4%) | 1336 (52.7%) |
| Smoker | |||
| Never | 2841 (79.5%) | 1368 (72.4%) | 1656 (65.4%) |
| Current/Past | 731 (20.5%) | 521 (27.6%) | 878 (34.6%) |
| Hypertension | |||
| No | 2782 (77.9%) | 1729 (91.5%) | 1806 (71.3%) |
| Yes | 790 (22.1%) | 160 (8.5%) | 728 (28.7%) |
| Abdominal radiation | |||
| No | 2661 (74.5%) | 1570 (83.1%) | 2042 (80.6%) |
| Yes | 911 (25.5%) | 319 (16.9%) | 492 (19.4%) |
| Total body irradiation | |||
| No | 3559 (99.6%) | 1875 (99.3%) | 2531 (99.9%) |
| Yes | 13 (0.4%) | 14 (0.7%) | 3 (0.1%) |
| Hypothalamic-pituitary axis radiation | |||
| No | 1278 (35.8%) | 1151 (60.9%) | 1146 (45.2%) |
| Yes | 2294 (64.2%) | 738 (39.1%) | 1388 (54.8%) |
| Nephrectomy | |||
| No | 3229 (90.4%) | 1736 (91.9%) | 2369 (93.5%) |
| Yes | 343 (9.6%) | 153 (8.1%) | 165 (6.5%) |
| Alkylating agents | |||
| No | 1816 (50.8%) | 849 (44.9%) | 1068 (42.1%) |
| Yes | 1756 (49.2%) | 1040 (55.1%) | 1466 (57.9%) |
| Heavy metals | |||
| No | 3406 (95.4%) | 1577 (83.5%) | 2231 (88.0%) |
| Yes | 166 (4.6%) | 312 (16.5%) | 303 (12.0%) |
| Age at cancer diagnosis (yrs.) | |||
| Median (IQR) | 7.7 (10.5) | 8.9 (9.9) | 7.7 (10.2) |
| Age at last contact (yrs.) | |||
| Median (IQR) | 41.5 (12.4) | 30.1 (8.5) | 35.5 (15.3) |
| Body mass index (kg/m2) | |||
| Median (IQR) | 22.8 (5.5) | 24.8 (6.9) | 26.4 (8.9) |
IQR, interquartile range; All the survivors are of European ancestry.
Age-standardized prevalence ratio of hypertension
The PRS-decile-specific age-standardized prevalence ratios of hypertension in the CCSS original cohort, CCSS expansion cohort and the SJLIFE are provided in Figure 1. Among survivors with the PRS in the top decile, the prevalence of hypertension was greater than expected in all three cohorts, with a prevalence ratio of 1.47 (CCSS original cohort), 1.03 (CCSS expansion cohort) and 2.07 (SJLIFE). Notably, in the clinically-assessed SJLIFE, the prevalence ratio was greater than 1 in all categories of the PRS.
Figure 1 |. Age-standardized prevalence ratios of hypertension by polygenic risk score (PRS) deciles.
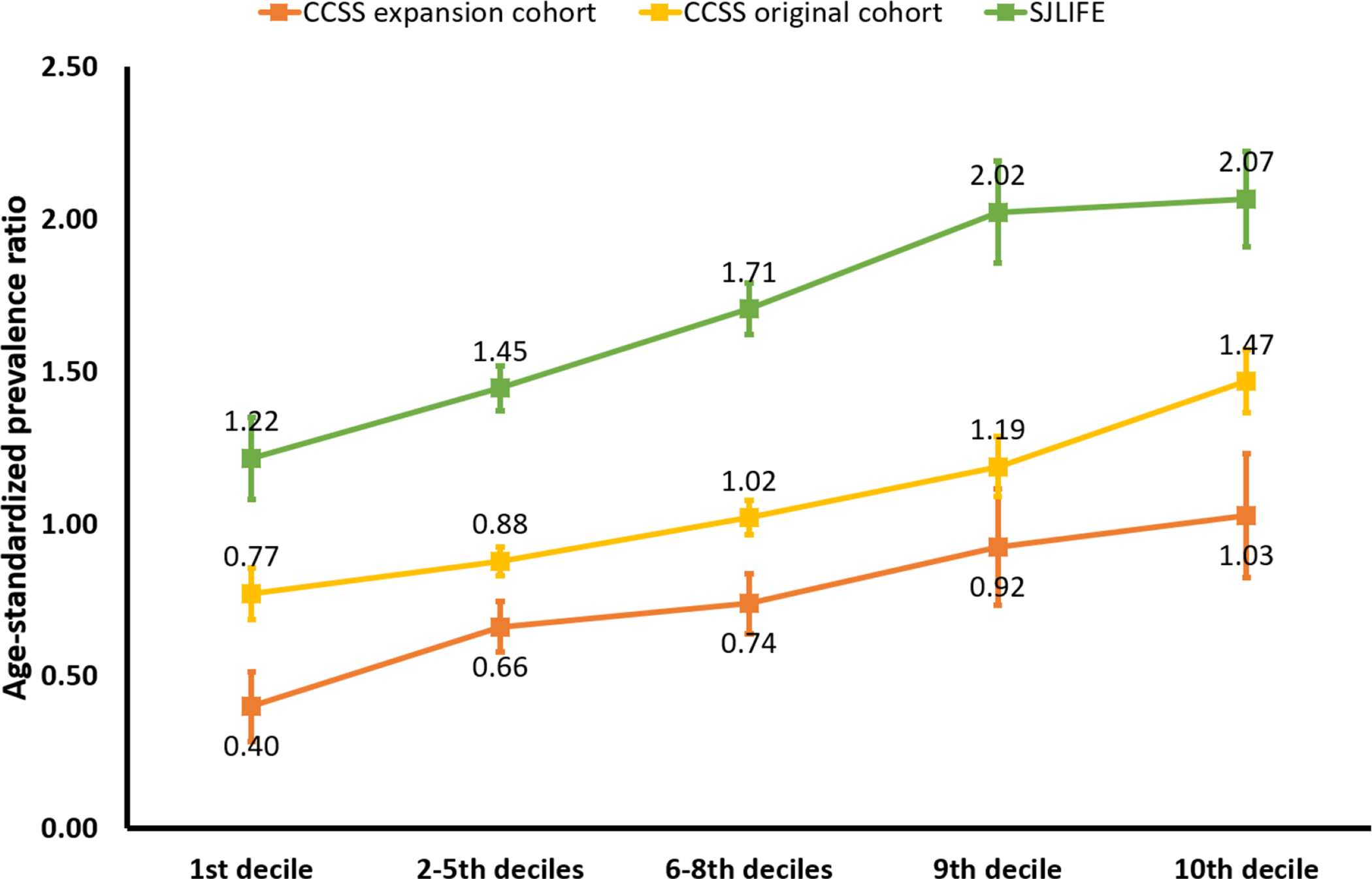
For survivors in the Childhood Cancer Survivor Study (CCSS) original and expanded cohorts (self-report of hypertension) and the St. Jude Lifetime Cohort study (clinical assessment of hypertension), the ratio of the observed number of hypertensive survivors in each individual cohort to the expected number was calculated. The nonparametric bootstrap with 1000 replicates was used to estimate the 95% confidence interval. Among survivors with the PRS in the top decile, the prevalence of hypertension was greater than expected in all three cohorts.
Association of the PRS with hypertension
In the CCSS original cohort, the PRS was significantly associated with an increased prevalence of hypertension (adjusted OR [aOR] per one SD of the PRS 1.31; 95% CI 1.20–1.42; P<0.001) (Table 2). Compared to survivors with the PRS in the lower decile, those in upper deciles had a greater prevalence of hypertension; for example, survivors in the top decile had a 2.63-fold higher odds (95% CI 1.80–3.83; P<0.001). Similar results were observed among survivors in the CCSS expansion cohort with a significantly increased prevalence of hypertension per SD change in the PRS (aOR=1.33; 95% CI=1.12–1.58; P<0.001) and top vs. bottom decile (aOR=3.03; 95% CI=1.39–6.60; P=0.005). The adjusted ORs of clinically assessed hypertension in the SJLIFE were also comparable for both per SD change in the PRS (aOR 1.39; 95% CI 1.26–1.54; P<0.001) and the top vs. bottom decile (aOR 2.60; 95% CI=1.66–4.06; P<0.001) than those in the CCSS cohorts with self-reported hypertension. In the combined sample of all three cohorts, the PRS remained consistently associated with hypertension [per SD change: aOR 1.34; 95% CI 1.26–1.43; P<0.001 and top vs. bottom decile aOR .66; 95% CI=2.03–3.48; P<0.001]. Survivors with the PRS in intermediate deciles also showed significantly increased prevalence of hypertension (aOR≥1.24) compared to those with the PRS in the bottom decile (Table 2).
Table 2 |.
Multivariable adjusted associations of the blood pressure PRS from the general population with hypertension among childhood cancer survivors
| PRS | CCSS Original cohort (790 hypertensive survivors; 2782 hypertension-free survivors) | CCSS Expansion cohort (160 hypertensive survivors; 1729 hypertension-free survivors) | SJLIFE (728 hypertensive survivors; 1806 hypertension-free survivors) | Combined sample (1678 hypertensive survivors; 6317 hypertension-free survivors) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | C-index | OR (95% CI) | P | C-index | OR (95% CI) | P | C-index | OR (95% CI) | P | C-index | |
| per SD change | 1.31 (1.20–1.42) | <0.001 | 0.736 | 1.33 (1.12–1.58) | <0.001 | 0.747 | 1.39 (1.26–1.54) | <0.001 | 0.788 | 1.34 (1.26–1.43) | <0.001 | 0.785 |
| 1st decile | Reference | |||||||||||
| 2–5th deciles | 1.17 (0.85–1.62) | 0.329 | 0.736 | 1.83 (0.93–3.63) | 0.082 | 0.745 | 1.18 (0.81–1.73) | 0.385 | 0.787 | 1.24 (0.99–1.56) | 0.066 | 0.784 |
| 6–8th deciles | 1.44 (1.04–2.00) | 0.030 | 1.89 (0.95–3.77) | 0.072 | 1.76 (1.20–2.58) | 0.004 | 1.61 (1.27–2.03) | <0.001 | ||||
| 9th decile | 1.89 (1.29–2.79) | 0.001 | 2.29 (1.03–5.10) | 0.042 | 2.32 (1.46–3.70) | <0.001 | 2.08 (1.58–2.75) | <0.001 | ||||
| 10th decile | 2.63 (1.80–3.83) | <0.001 | 3.03 (1.39–6.60) | 0.005 | 2.60 (1.66–4.06) | <0.001 | 2.66 (2.03–3.48) | <0.001 | ||||
PRS, polygenic risk score derived from 895 blood pressure loci in Evangelou et al.(13); SD, standard deviation; OR, odds ratio; CI, confidence interval; Covariates included age at childhood cancer diagnosis, age at last follow-up, sex, body mass index, smoking status (current or former vs. never), physical activity (vigorous intensity exercise in metabolic equivalents), cohort (CCSS original cohort, CCSS expansion cohort and SJLIFE), alkylating agents, heavy metals, abdominal radiation, hypothalamic-pituitary axis radiation, total body radiation and nephrectomy
The PRS showed greater effect in overweight/obese survivors (per SD change in PRS aOR 1.39; top decile vs. bottom decile aOR 3.51) than those with normal weight (per SD change in PRS aOR 1.23; top vs. bottom decile aOR 2.07) (Supplementary Table 2). These adjusted odds ratios were significantly different from each other (the interaction aOR per SD change in the PRS 1.13 (P=0.041); top decile vs. bottom decile aOR 1.85 (P=0.001) (Table 3). We did not observe a significant modification of the effect of the PRS in underweight versus normal weight survivors (Supplementary Table 2). A greater PRS effect was also observed among survivors exposed to HPA radiation (per SD change in PRS aOR 1.41; top decile vs. bottom decile aOR 3.07) compared to unexposed survivors (per SD change in PRS aOR 1.24; top vs. bottom decile aOR 2.13) [the interaction aOR per SD change in the PRS=1.18; P=0.005 and top vs. bottom decile aOR=1.44; P=0.195] (Supplementary Table 3). A slightly greater effect of the PRS on hypertension was observed among survivors with nephrectomy or exposed to abdominal radiation than unexposed survivors. However, no statistically significant interaction was found between the PRS and exposure to alkylating or platinum agents, abdominal radiation or nephrectomy (Supplementary Table 3).
Table 3 |.
Interaction of blood pressure PRS from the general population with body mass index and exposure to hypothalamic-pituitary axis radiation in hypertension risk among childhood cancer survivors
| PRS | PRS× Overweight/obese | PRS× Hypothalamic-pituitary axis radiation | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| per SD change | 1.13 (1.01–1.27) | 0.041 | 1.18 (1.05–1.33) | 0.005 |
| 1st decile | Reference (1.0) | |||
| 2–5th deciles | 1.21 (0.95–1.55) | 0.120 | 1.02 (0.64–1.63) | 0.930 |
| 6–8th deciles | 1.21 (0.94–1.55) | 0.140 | 1.23 (0.77–1.97) | 0.395 |
| 9th decile | 1.39 (0.93–2.06) | 0.104 | 1.45 (0.83–2.53) | 0.197 |
| 10th decile | 1.85 (1.27–2.69) | 0.001 | 1.44 (0.83–2.49) | 0.195 |
PRS, polygenic risk score derived from 895 blood pressure loci reported in Evangelou et al.(13); SD, standard deviation; OR, odds ratio; CI, confidence interval; Covariates included age at childhood cancer diagnosis, age at last follow-up, sex, body mass index, smoking status (current or former vs. never), physical activity (vigorous intensity exercise in metabolic equivalents), cohort (CCSS original cohort, CCSS expansion cohort and SJLIFE), alkylating agents, heavy metals, abdominal radiation, hypothalamic-pituitary axis radiation, total body radiation and nephrectomy
Attributable fraction of the PRS and cancer therapies for hypertension
The adjusted AF for hypertension due to both the PRS and the specific cancer therapies considered as risk factors by the COG guidelines was 40.2% (Central Illustration). This total AF can be broken down into three components: 21.0% attributed to the PRS; 15.7% attributable to cancer therapies; and the remaining 3.5% due to the joint effect of both cancer therapies and the PRS together, exceeding the two individual effects. The AF for the PRS and cancer therapies was greater for survivors who were underweight (54.6%), had normal weight (46.7%) or were <35 years (49.5%), and these increases were mainly due to greater contribution of cancer therapies in these survivors. The contribution of the PRS remained consistent across the subgroups based on body mass index, age and exposure to HPA radiation.
Central Illustration |. Adjusted attributable fraction (aAF) for hypertension by specific cancer therapies considered as risk factors by the Children’s Oncology Group guidelines and the polygenic risk score (PRS) among survivors.
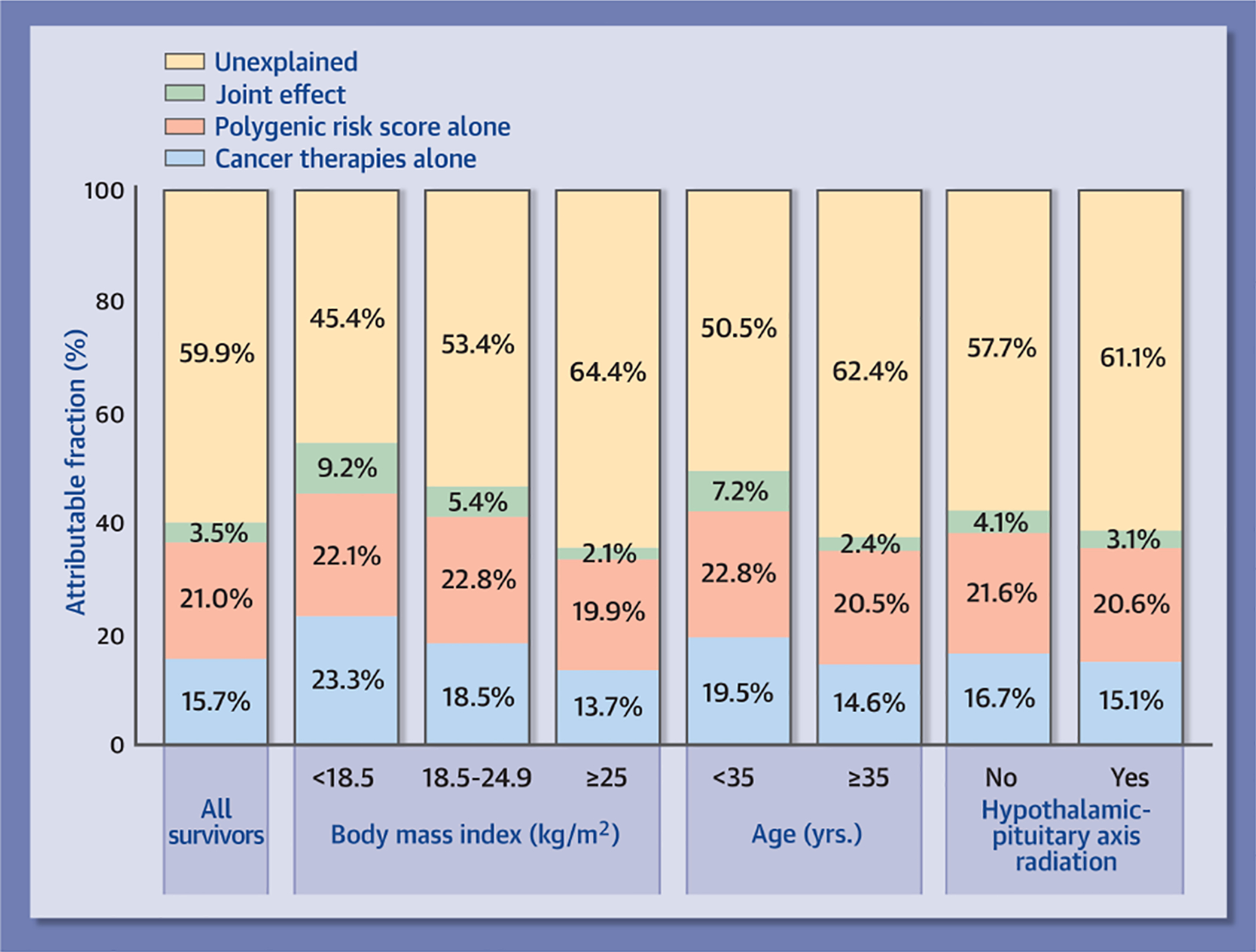
The aAF for hypertension due to both PRS and the specific cancer therapies was 40.2% (21.0% attributed to the PRS; 15.7% attributable to cancer therapies; and the remaining 3.5% due to the joint effect of both cancer therapies and the PRS together, exceeding the individual effects of the two). The contribution of the PRS remained generally consistent across the subgroups based on body mass index, age and exposure to hypothalamic-pituitary axis radiation.
Discussion
To our knowledge, this is the first study to apply and comprehensively evaluate the polygenic risk of blood pressure loci from the general population to childhood cancer survivors who are known to be at significantly greater risk of hypertension. Our results showed that survivors with the PRS in the top decile have a 2.7-fold increased odds of hypertension prevalence compared to those with the PRS in the bottom decile, and this risk was further exacerbated among overweight/obese survivors and those exposed to HPA radiation. Notably, our data indicate that the PRS and cancer therapies together accounted for 40.2% of hypertension among survivors, with the PRS alone contributing 21.0%.
We found a slightly attenuated PRS effect on hypertension among childhood cancer survivors compared to the 3.3-fold odds of hypertension prevalence among individuals with the PRS in the top decile in the general population(13). However, considering the absolute risk of hypertension in survivors is higher than those in the general population, the true effect of PRS among survivors of childhood cancer may be slightly greater than the observed 2.7-fold odds of hypertension prevalence. Thus, even a small increase in relative risk by the PRS can translate to substantial increase in absolute risk. Furthermore, the polygenic risk of hypertension was increased by being overweight/obese– a major late effect in childhood cancer survivors(26) and exposure to HPA radiation which is a risk factor for obesity in survivors(27). However, the attributable fraction due to the PRS was slightly lower among overweight/obese survivors (19.9%) compared to those with normal weight (22.8%). These seemingly-contradictory observations may be explained by the following: if the genetic contribution to the total risk is lower in presence of a strong non-genetic risk factor (overweight/obesity), the risk increase by the genetic factor is stronger because the baseline propensity for hypertension is elevated by the strong non-genetic strong risk factor.
An important finding in this study is that 40.2% of hypertension among survivors could be attributed to cancer therapies plus the PRS. About 16% of hypertension among survivors were attributed to cancer therapies and this contribution was higher (49% to 55%) among younger (<35 years) survivors or those with normal or underweight. Approximately 21% of hypertension among survivors could be attributed to the polygenic risk, which remained generally consistent with respect to body mass index, age and HPA radiation. Given that these estimates are independent of demographic, lifestyle and treatment factors and that the polygenic risk is established at birth, survivors with a high blood pressure polygenic risk may benefit from earlier screening and intervention measures to reduce the risk of hypertension and comorbidities. Additionally, evaluating the polygenic risk requires a one-time genetic test (currently <$100), highlighting the feasibility of genetic risk assessment. Our results, therefore, suggest that consideration of blood pressure polygenic risk in combination with established cancer treatment-related risk factors can identify survivors at high risk of hypertension. Genetic testing for polygenic risk, in concert with assessment of clinical risk factors, could be used to risk stratify frequency of ongoing follow-up in normotensive survivors at high risk and therapeutic interventions (e.g., lifestyle, pharmacologic) for those with elevated blood pressure or established hypertension.
Limitations should be considered when interpreting the results of this study. While the PRS and cancer therapies explained approximately 40% of hypertension among survivors, further investigations are needed to understand the causes of remaining 60% hypertensive survivors. In the CCSS survivors, hypertension was diagnosed based on self-report, which may be prone to misclassification and hence the reported blood pressure PRS effect on hypertension may be underestimated. Clinically-assessed cohorts of childhood cancer survivors such as the SJLIFE provide a more accurate estimate of the PRS. Other such cohorts with both genetic and outcome data are not currently available, however. Kidney abnormalities are known predictors of hypertension; however, we were unable to assess their potential influence on the PRS-hypertension association because of limitations in self-reported data in the CCSS, which comprises approximately 68% of the study population. Nonetheless, the genotypes are independent of these potential confounders, and if not, they would be in the pathway that the PRS, thus capturing their risk at least partially. The PRS could be calculated only for five-year childhood cancer survivors who provided DNA samples in SJLIFE and CCSS. Thus, our findings may not be generalizable to cancer patients who died before reaching the five-year milestone. Our analyses were also restricted to survivors of European ancestry to be consistent with the populations used to derive the existing PRS. There is a need to assess the polygenic risk among survivors of non-European ancestry.
Conclusions
In conclusion, we found that the blood pressure PRS from the general population predicts hypertension among childhood cancer survivors in three independent cohorts. Survivors with higher polygenic risk were at significantly increased risk of hypertension, independent of demographic, lifestyle and treatment risk factors, and this risk was further elevated among overweight/obese survivors and those exposed to HPA radiation. Approximately one quarter of the hypertensive survivors were attributed to the PRS alone; together with the cancer therapies, this contribution increased to over 40%. Our results, therefore, have strong implications for the personalized management of current survivors of childhood cancer and future cancer patients. While screening for hypertension in all childhood cancer survivors is important, enhanced awareness and prevention are warranted for those who are at higher risk due to the PRS, clinical factors, and treatment exposures.
Supplementary Material
Clinical Perspectives.
Competencies in Medical Knowledge:
The prevalence of hypertension in childhood cancer survivors is over 2.5 times higher than in non-cancer survivor controls. Importantly, survivors exposed to cardiotoxic cancer therapy have been shown to be at increased risk of cardiomyopathy, but those who also develop hypertension experience a near multiplicative increase in risk of cardiomyopathy. A blood pressure polygenic risk score (PRS) established in the general population adds significantly to the prediction of high risk subgroups for hypertension in childhood cancer survivors of European ancestry. Specifically, the attributable fraction of hypertension to PRS and cancer treatments together is over 40%. Compared to survivors in the lower 10% of the PRS, survivors in all the other deciles of the PRS are at increased risk of hypertension, independent of demographic, lifestyle and treatment risk factors. This PRS associated risk was further elevated among survivors exposed to hypothalamic-pituitary axis radiation and those who were overweight/obese.
Translational Outlook:
While screening for hypertension in all childhood cancer survivors is important, enhanced awareness and prevention are warranted for those who are at higher risk due to the PRS, clinical factors, and treatment exposures. Future research should be focused on the evaluation of genetic testing and application of polygenic risk scores, in concert with the assessment of clinical risk factors, to risk stratify frequency of ongoing follow-up in normotensive survivors at high risk and determine clinically appropriate therapeutic interventions (e.g., lifestyle, pharmacologic) for those with elevated blood pressure or established hypertension.
Funding:
The St. Jude Lifetime Cohort (SJLIFE; U01 CA195547: MMH and LLR, Principal Investigators) and the Childhood Cancer Survivor Study (CCSS; U24 CA55727; GTA Principal Investigator) are supported by the National Cancer Institute at the National Institutes of Health and the Cancer Center Support CORE grant (CA21765: C. Roberts, Principal Investigator). The CCSS original cohort genotyping was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. This work is also supported by R01 CA216354 (YY and JZ, Principal Investigators) from the National Cancer Institute at the National Institutes of Health and the American Lebanese Syrian Associated Charities, Memphis, Tennessee.
Abbreviations:
- PRS
Polygenic risk score
- SNPs
Single nucleotide polymorphisms
- GWAS
Genome-wide association study
- CCSS
Childhood Cancer Survivor Study
- SJLIFE
The St. Jude Lifetime Cohort
- COG
Children’s Oncology Group
- HPA
Hypothalamic-pituitary axis
- SJCRH
St. Jude Children’s Research Hospital
- QC
Quality control
- WGS
Whole-genome sequencing
- CTCAE
the National Cancer Institute’s Common Terminology Criteria for Adverse Events
- IQRs
interquartile ranges
- OR
Odds ratio
- CI
Confidence interval
- SD
Standard deviation
- AF
Attributable fraction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
References
- 1.Armstrong GT, Chen Y, Yasui Y et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. New Engl J Med 2016;374:833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulrooney DA, Armstrong GT, Huang S et al. Cardiac Outcomes in Adult Survivors of Childhood Cancer Exposed to Cardiotoxic Therapy: A Cross-sectional Study. Ann Intern Med 2016;164:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulrooney DA, Yeazel MW, Kawashima T et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009;339:b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006;355:1572–82. [DOI] [PubMed] [Google Scholar]
- 5.Bhakta N, Liu Q, Ness KK et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet 2017;390:2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mertens AC, Yasui Y, Neglia JP et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol 2001;19:3163–72. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong GT, Oeffinger KC, Chen Y et al. Modifiable Risk Factors and Major Cardiac Events Among Adult Survivors of Childhood Cancer. Journal of Clinical Oncology 2013;31:3673–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armenian SH, Xu LF, Ky B et al. Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. Journal of Clinical Oncology 2016;34:1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow EJ, Baker KS, Lee SJ et al. Influence of Conventional Cardiovascular Risk Factors and Lifestyle Characteristics on Cardiovascular Disease After Hematopoietic Cell Transplantation. Journal of Clinical Oncology 2014;32:191–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson TM, Li Z, Green DM et al. Blood Pressure Status in Adult Survivors of Childhood Cancer: A Report from the St. Jude Lifetime Cohort Study. Cancer Epidemiol Biomarkers Prev 2017;26:1705–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers, Version 5.0. Monrovia, CA: Children’s Oncology Group; October 2018. [Google Scholar]
- 12.Hudson MM, Ness KK, Gurney JG et al. Clinical Ascertainment of Health Outcomes Among Adults Treated for Childhood Cancer. JAMA 2013;309:2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evangelou E, Warren HR, Mosen-Ansorena D et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet 2018;50:1412–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robison LL, Armstrong GT, Boice JD et al. The Childhood Cancer Survivor Study: A National Cancer Institute-Supported Resource for Outcome and Intervention Research. Journal of Clinical Oncology 2009;27:2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robison LL, Mertens AC, Boice JD et al. Study design and cohort characteristics of the childhood cancer survivor study: A multi-institutional collaborative project. Med Pediatr Oncol 2002;38:229–239. [DOI] [PubMed] [Google Scholar]
- 16.Hudson MM, Ness KK, Nolan VG et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer 2011;56:825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morton LM, Sampson JN, Armstrong GT et al. Genome-Wide Association Study to Identify Susceptibility Loci That Modify Radiation-Related Risk for Breast Cancer After Childhood Cancer. Jnci-J Natl Cancer I 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sapkota Y, Turcotte LM, Ehrhardt MJ et al. Genome-Wide Association Study in Irradiated Childhood Cancer Survivors Identifies HTR2A for Subsequent Basal Cell Carcinoma. J Invest Dermatol 2019;139:2042–2045 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das S, Forer L, Schonherr S et al. Next-generation genotype imputation service and methods. Nat Genet 2016;48:1284–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sapkota Y, Cheung YT, Moon W et al. Whole-Genome Sequencing of Childhood Cancer Survivors Treated with Cranial Radiation Therapy Identifies 5p15.33 Locus for Stroke: A Report from the St. Jude Lifetime Cohort Study. Clin Cancer Res 2019;25:6700–6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sapkota Y, Wilson CL, Zaidi AK et al. A novel locus predicts spermatogenic recovery among childhood cancer survivors exposed to alkylating agents. Cancer Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang ZM, Wilson CL, Easton J et al. Genetic Risk for Subsequent Neoplasms Among Long-Term Survivors of Childhood Cancer. Journal of Clinical Oncology 2018;36:2078–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang CC, Chow CC, Tellier LCAM, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudson MM, Ehrhardt MJ, Bhakta N et al. Approach for Classification and Severity Grading of Long-term and Late-Onset Health Events among Childhood Cancer Survivors in the St. Jude Lifetime Cohort. Cancer Epidem Biomar 2017;26:666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taguri M, Kuchiba A. Decomposition of the population attributable fraction for two exposures. Ann Epidemiol 2018;28:331–334 e1. [DOI] [PubMed] [Google Scholar]
- 26.Wilson CL, Liu W, Yang JJ et al. Genetic and clinical factors associated with obesity among adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort. Cancer-Am Cancer Soc 2015;121:2262–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lustig RH, Hinds PS, Ringwald-Smith K et al. Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab 2003;88:2586–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


