Fig. 2.
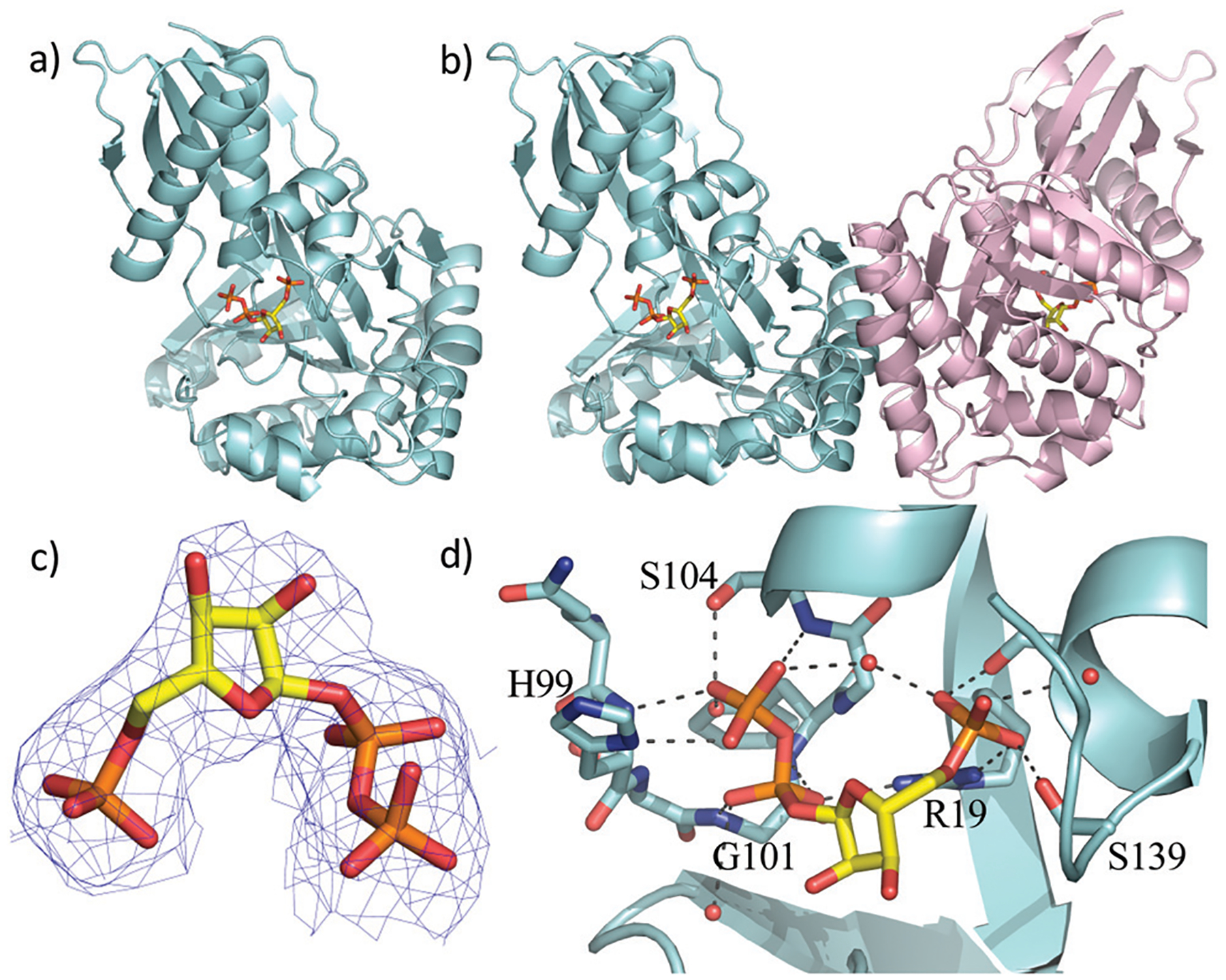
X-Ray crystal structure of the WT ForT/PRPP complex. (a) The ForT monomer shown as a cyan cartoon, PRPP, shown in sticks (carbon yellow, phosphorous orange, oxygen red) is at the centre of the monomer. (b) The ForT dimer is generated by crystal symmetry, the second monomer is pale pink cartoon, PRPP is remote from the dimer interface. (c) The final 2Fo–Fc map contoured at 1.2σ for PRPP (shown as in Fig. 2a) (the original Fo–Fc map is shown in Fig. S3, ESI†). (d) PRPP is bound to the enzyme by an extensive array of hydrogen bonds. The loop (Gln-98 to Ser-108), characteristic of GHMP kinase superfamily, plays a crucial role in substrate binding. Protein carbon atoms are coloured in cyan, nitrogen in blue, other atoms as in Fig. 2a.
