Abstract
Background:
The COVID-19 pandemic has induced historic educational disruptions. In April 2021, about 40% of U.S. public school students were not offered full-time in-person education.
Objective:
To assess the risk for SARS-CoV-2 transmission in schools.
Design:
An agent-based network model was developed to simulate transmission in elementary and high school communities, including home, school, and interhousehold interactions.
Setting:
School structure was parametrized to reflect average U.S. classrooms, with elementary schools of 638 students and high schools of 1451 students. Daily local incidence was varied from 1 to 100 cases per 100 000 persons.
Participants:
Students, faculty, staff, and adult household members.
Intervention:
Isolation of symptomatic individuals, quarantine of an infected individual’s contacts, reduced class sizes, alternative schedules, staff vaccination, and weekly asymptomatic screening.
Measurements:
Transmission was projected among students, staff, and families after a single infection in school and over an 8-week quarter, contingent on local incidence.
Results:
School transmission varies according to student age and local incidence and is substantially reduced with mitigation measures. Nevertheless, when transmission occurs, it may be difficult to detect without regular testing because of the subclinical nature of most children’s infections. Teacher vaccination can reduce transmission to staff, and asymptomatic screening improves understanding of local circumstances and reduces transmission.
Limitation:
Uncertainty exists about the susceptibility and infectiousness of children, and precision is low regarding the effectiveness of specific countermeasures, particularly with new variants.
Conclusion:
With controlled community transmission and moderate mitigation, elementary schools can open safety, but high schools require more intensive mitigation. Asymptomatic screening can facilitate reopening at higher local incidence while minimizing transmission risk.
Primary Funding Source:
Centers for Disease Control and Prevention through the Council of State and Territorial Epidemiologists, National Institute of Allergy and Infectious Diseases, National Institute on Drug Abuse, and Facebook.
During the spring 2020 outbreak of COVID-19, all 50 states recommended or mandated public school closures, affecting at least 124 000 U.S. schools and 55.1 million students (1). Reopening has proven challenging, and as of April 2021, only about 60% of students in kindergarten through 12th grade (K-12) were offered full-time, in-person learning (2). This percentage has nearly doubled since December 2020; however, where in-person education is offered, many families continue to opt out, including 43% in Boston Public Schools and 65% in New York City Public Schools (3, 4). Nevertheless, many parents and advocates have objected strongly to school closures (5), and the Biden administration has stated a priority to facilitate safe, in-person school reopening (6).
Debates around school reopening have been heated and often invoke seemingly contradictory evidence about safety. For example, many well-studied cases in school settings have found minimal secondary transmission (7–9). Nevertheless, school clusters have also been documented, particularly in Israel and parts of the United States (10–12), and some observational studies have suggested that school closures may have substantially reduced transmission (13, 14). Proponents of reopening schools point to evidence that COVID-19 is most often mild in children (15), although others have expressed concern about transmission to staff, families, and the community. The discord plays out similarly on a macro scale: Many Asian and European countries reopened schools with physical distancing when community transmission was low and reported negligible increases in transmission (16, 17). Some, including France, the United Kingdom, and Ireland, kept schools open during the fall wave and reversed surging transmission by closing other sectors, although additional closures have occurred since the emergence of variant B.1.1.7 (18, 19). Other countries, such as Austria, the Czech Republic, and South Korea, closed schools to address rising case burdens (18).
Nevertheless, there is little debate that the benefits of in-person education are substantial, particularly amid reports of high levels of remote absenteeism; increased depression, anxiety, and suicidality; and parent concerns around educational quality (20–22). Beyond educational and mental health outcomes, opening schools also improves access to social services for children and labor market outcomes for working parents, especially women (23–27). To reopen safely, it is critical to take a comprehensive account of the full array of evidence and identify procedures to minimize risks.
We simulated SARS-CoV-2 transmission dynamics in elementary and high schools, characterizing how school transmission may occur as either isolated or sustained outbreaks. Although several recent articles have discussed the effect of school reopening on local transmission under different mitigation strategies (28–30), our work focused on the risk that transmission will occur on a school campus and spread to household members (31, 32). We emphasized uncertainty in transmission risk (rather than focusing on the average) as well as the observability of infections to school and public health staff, reconciling apparent contradictions in evidence. We evaluated outcomes under varying combinations of local incidence, in-classroom mitigation efforts, testing practices, and staff vaccination.
Methods
Analytic Overview
We developed an age-specific, agent-based network model of COVID-19 transmission in elementary and high school communities (Appendix Figure). We incorporated interactions within schools and homes, as well as those between households outside school. Because data are inconclusive on transmission among middle school–aged children, we did not model middle schools explicitly; accumulating evidence suggests that middle schoolers may be more similar to high schoolers than elementary schoolers with respect to susceptibility and infectiousness (33, 34).
Strategies
We simulated combinations of interventions, incorporating strategies that target the following 3 axes: general infection control in schools, COVID-19–specific countermeasures, and scheduling and cohorting. Parameters for the model and strategies are detailed in the Appendix Table.
General Infection Control in Schools
Low uptake.
Schools implemented no or minimal general infection control measures, such as masking and distancing.
Medium uptake.
Schools implemented masking and distancing, and adherence was moderate, such that the risk for transmission given infectious contact was two thirds that in a school with low uptake.
High uptake.
Schools implemented masking and distancing, and adherence was high, such that the risk for transmission given infectious contact was one third that in a school with low uptake.
COVID-19–Specific Countermeasures
Symptom-based self-isolation (“symptomatic isolation”).
Individuals in the school were screened for symptoms daily, and those who developed clinically recognizable symptoms did not attend school. In setting guidance for symptom-based isolation, schools balance between expansive symptom definitions that lead to many unnecessary missed days and more restrictive definitions that may miss subtle presentations of COVID-19 (55, 56). Our primary source for this parameter defined symptoms triggering isolation as fever; respiratory illness (such as cough or shortness of breath); gastrointestinal symptoms; and new loss of smell or taste, which affect about 20% of child and adolescent cases (37).
Diagnostic testing plus classroom notification (“classroom quarantine”).
Individuals who developed symptoms were isolated and immediately tested. The school received results within a day, and all classrooms associated with a person who tested positive were notified and closed for 10 days, following guidelines from the Centers for Disease Control and Prevention (34).
Teacher vaccination with an infection-blocking vaccine (“staff vaccination”).
In addition to classroom quarantine, teacher susceptibility was reduced to 33% of baseline, roughly assuming that 75% of teachers receive an infection-blocking vaccine with 90% effectiveness (48, 49, 57).
Weekly asymptomatic screening (“weekly screening”).
In addition to classroom quarantine, asymptomatic students and staff at each school were tested weekly to reduce asymptomatic and presymptomatic transmission. We assumed that schools used a polymerase chain reaction test with either saliva or a swab of the anterior nares and that results were available within 24 hours. We further specified 90% uptake and 90% test sensitivity. This reflected the sensitivity of polymerase chain reaction tests, although some studies also suggest that antigen tests may reach 90% sensitivity during the infectious period (with a faster turnaround time) (50–54). On receipt of a positive result, infected individuals isolated outside school for 10 days on the basis of guidelines from the Centers for Disease Control and Prevention, and siblings and classroom members of a person who tested positive were notified and quarantined for 10 days.
Scheduling and Cohorting
Five-day schedule.
This scenario simulated a traditional 5-day, in-person learning schedule, allowing for classroom contacts, brief staff–staff interactions (10 per day), and random contacts between school members (20 per day). Related arts and special education teachers had revolving contact with 5 classrooms per day.
Cohorting.
This scenario again assumed 5 days of in-person learning for all students, but with restricted out-of-classroom contacts, including separation of classes for lunch and recess. Students and primary teachers continued to have sustained classroom contacts. We assumed a 50% decrease in the number of out-of-classroom contacts during the school day and remote teaching of related arts.
Half class sizes.
All students attended school 5 days per week but in classes of half their typical size. To accommodate this, the number of teachers was doubled. Limited contacts as in the cohorting strategy were also maintained.
Hybrid (A/B) scheduling.
Classes were subdivided into 2 cohorts, and students attended school for 2 days per week, either Monday and Wednesday or Tuesday and Thursday. Elementary school children in the same household attending the same school were sorted into the same cohort. All staff were physically present each instructional day. In sensitivity analyses, we considered alternative hybrid schedules. Limited contacts as in the cohorting strategy were also maintained.
Model Structure
We generated a set of synthetic households from U.S. population data (58), including students, staff, and adult household members (Supplement). We simulated an average-sized elementary school of 638 pupils from 492 households, of which 134 had more than 1 child in the school. We set 5 classes per grade level, an average class size of 21 children, and 1 teacher per class. We also incorporated 30 additional staff to reflect such roles as administrators, counselors, cafeteria staff, custodians, special education teachers, and teachers of related arts (or “specials,” such as music and art). These were assigned either rotating in-classroom roles (n = 15) or out-of-classroom roles (n = 15).
We simulated an average-sized high school of 1451 students in 1223 households, of which 210 had more than 1 student in the school (35). Students rotated among 8 class periods per day (23 students and 1 teacher), and the distribution of students to class periods was chosen randomly within grade levels but repeated daily for the full simulation. The high school had 45 additional staff with out-of-classroom roles and 15 with in-classroom roles. In both elementary and high schools, teachers were modeled to have additional staff contacts per day (for example, contacts in break rooms or offices).
To reflect social interactions and out-of-school childcare, our base case assumed that each family interacted with 1 additional family on each day they did not attend school, randomly reassorted each day. We varied this number in sensitivity analyses from 0 to 9 families per day out of school. We assumed that when families mix, no more than 2 adults attend at a given time, who are randomly selected.
Transmission
At each daily time step, we modeled dyadic interactions between individuals according to household, classroom, school, and childcare relationships, drawing parameter values from the distributions specified in the Appendix Table. A person infected with SARS-CoV-2 transmitted to susceptible individuals according to contact type and length (such as home or school), infectiousness and susceptibility (adult vs. child), symptom status, and an individual dispersion factor.
Secondary Attack Rate
The secondary attack rate for SARS-CoV-2 (the probability that a person infected with SARS-CoV-2 transmits it to a person they contact) varies by contact type—in this case, household, classroom, random school, and out-of-school social or childcare contacts. Although household attack rates vary substantially across geographic locations, corresponding to cultural norms and precautions adopted, we assumed, based on 2 meta-analyses, a household adult–adult secondary attack rate of 20% over the full duration of an infection, translating to approximately 4% per day (38, 39).
For school-based transmission, we allowed full school-day adult–adult attack rates from symptomatic infections to reach up to 3% per day for scenarios with low uptake of masks and distancing, a downward adjustment from the household attack rate to account for less close contact in professional settings. Transmission was further scaled to reflect duration of contact, symptom status, and mitigation. We developed the scale of reductions in transmission from mitigation measures in line with observations from household settings with high prevention measures (33, 59) and based on effectiveness analyses for measures like masks (60, 61). We discuss comparisons to observed SARs and outbreaks in the Supplement.
Relative Susceptibility and Infectiousness in Children
Household contact tracing studies suggest that adults are likely more susceptible to COVID-19 and more apt to transmit it when infected than children, although data, particularly for the latter, are equivocal and limited by availability and timing of testing (62). Several sources indicate that any differences wane by the teenage years, possibly as early as age 10 (8, 10, 33, 63, 64). We assumed that elementary school children were half as susceptible as adults and that high school children and adults were equally susceptible. Data are similarly limited to inform transmission from children with COVID-19. We further specified that elementary school children were half as infectious as adults and high school students were equally as infectious as adults (33, 63). We discuss the data underlying these assumptions in the Supplement, and we varied these assumptions in sensitivity analyses.
Asymptomatic Transmission and Overdispersion
We assumed that persons with fully asymptomatic disease transmit COVID-19 at half the rate of those with any symptoms (36). Those who had mild, subclinical symptoms but were not fully asymptomatic transmitted at the same rate as those with clinical symptoms, although only the latter were assumed to self-isolate. Although reported heterogeneity in transmission may be partially driven by differences in contact rates, we sampled adult transmissibility according to a lognormal distribution to allow for some variation by individual characteristics (such as viral load) (40, 41).
Implementation and Clinical Outcomes
We first evaluated the downstream effect of a single infectious person. We randomly designated 1 member of the school (student or staff) as infected, starting on a random day of the week. We assessed the spread of the virus over 30 days because in most cases either all infections were resolved over that time horizon or the spread was sufficiently large that additional public health measures (such as school closures) would likely be adopted. For each scenario, we ran our model 2000 times and summarized the mean number of infections generated in the school over 30 days after the index case, the percentage of scenarios without transmission from the index case, and the percentage of scenarios with more than 5 in-school transmissions. We also describe the composition of secondary cases (proportion occurring in students, staff, and family members of students or staff).
Second, we quantified SARS-CoV-2 infections among the school community across a typical school quarter, given a constant local incidence. On each day over 8 weeks, every susceptible person had a probability of becoming spontaneously infected outside school that was equivalent to a community per capita daily incidence adjusted for age (with children and adolescents at half the probability of adults), distinct from their contact-dependent risk within the school community. Local incidence is intended to reflect reported case counts, and thus we assumed that cases are underreported by a factor of 3 (65). When schools were remote, we assumed (similar to the analysis of hybrid schedules) that each family interacted with 1 additional family on each day they did not attend school. For each scenario, we summarized cumulative incidence, as well as incremental incidence compared with remote learning.
We defined a reopening strategy as controlling transmission in a group (students, educators or staff, or families) if it resulted on average in less than a 1-point increase in the percentage of the group that was infected, compared with remote learning. This threshold is consistent with thresholds used by similar studies, with the objective of minimal in-school transmission (30, 66).
The model was implemented in R, version 4.0.2 (R Foundation). Model code is publicly available as an R package at https://github.com/abilinski/BackToSchool2.
Role of the Funding Source
The funders had no role in the design or conduct of this study or in the decision to submit this work for publication.
Results
In-School Mitigation
In elementary schools with low mitigation and classroom quarantine under a 5-day schedule, we projected an average of 1.7 secondary cases over 30 days after infection of a single index case patient (Figure 1, top left). This decreased to 0.9 cases with medium mitigation and further to 0.3 cases with high mitigation. Under classroom quarantine, transmission was most reduced by replacing the 5-day schedule with an A/B schedule (to between 0.1 and 0.4 secondary cases depending on uptake of in-school prevention). With high mitigation, an average of less than 0.5 secondary transmissions occurred per case over 30 days in all scenarios.
Figure 1. Average number of total secondary transmissions over 30 days (outside the index case patient’s household) after a single introduction into a school.
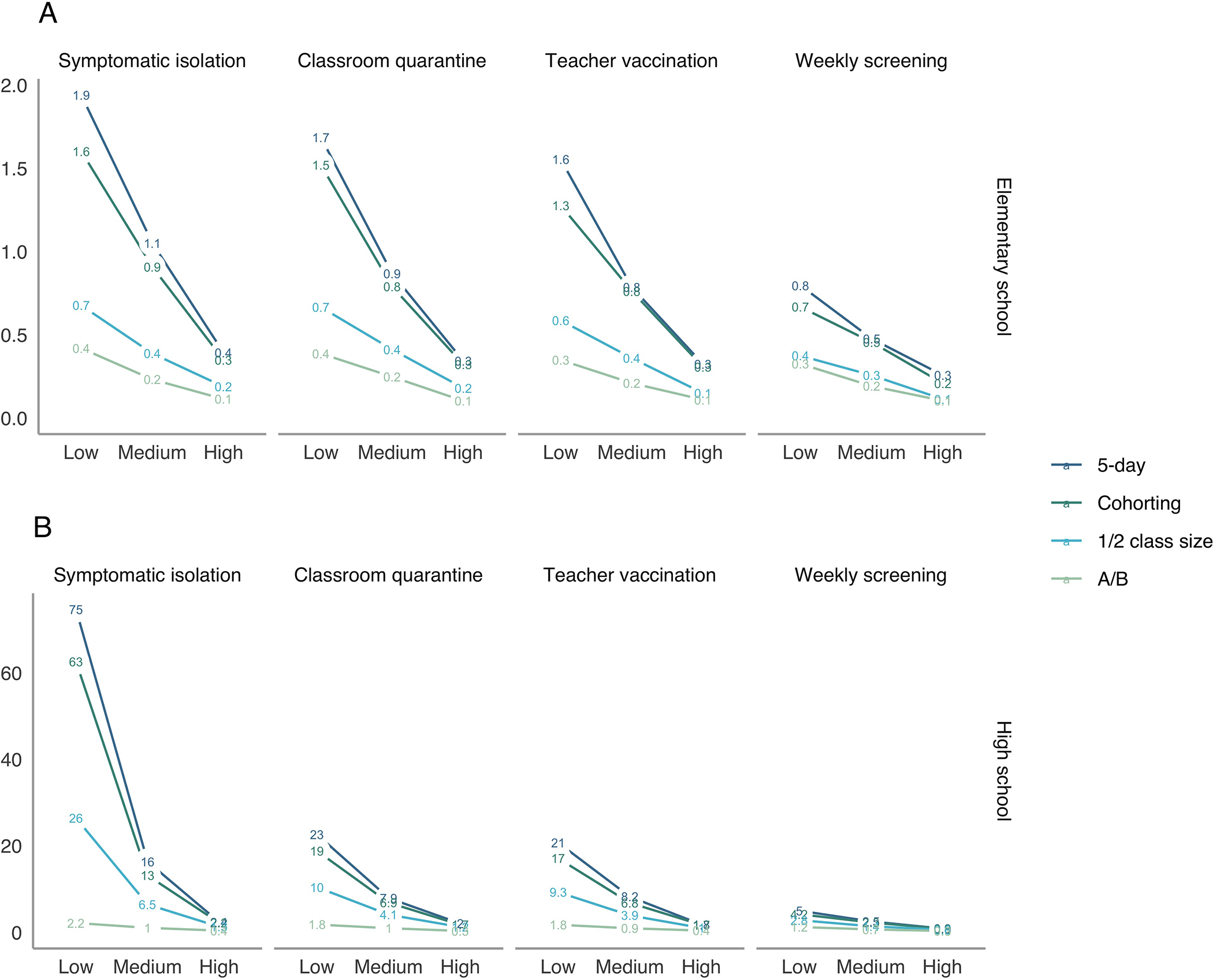
This figure displays the average number of secondary transmissions over 30 days following a single case introduction into a school. Estimates include both transmission directly from the index case and that from secondary and tertiary cases. The top panel shows elementary schools, where children are assumed to be less susceptible and less infectious than adults, and the bottom panel shows high schools. Note that axes differ across rows. The x-axes vary the level in-school mitigation with “low” assuming minimal interventions and “high” assuming intensive interventions. A/B = hybrid. (This is not an estimate of the effective reproduction number [Rt], which is shown in Supplement Figures 9 and 10.)
In high schools, we found greater potential for larger outbreaks after a single introduction into the school, particularly when uptake of in-school prevention was low (Figure 1, bottom). For example, with low mitigation and classroom quarantine under a 5-day schedule, we projected 23 secondary cases in the school community over a 30-day period (in the absence of additional public health responses like school closure). High uptake of in-school mitigation reduced this to 2.0 cases.
Quarantine, Teacher Vaccination, and Screening
In elementary schools, classroom quarantine had a modest effect on transmission, although its effect was greater if children were as infectious as adults (Supplement Figure 1). In high schools, classroom quarantine reduced projected average transmissions by a factor of 0.3 under low mitigation but only by 0.86 with high mitigation. We found a small effect of teacher vaccination on overall transmission but a substantial effect on transmission to teachers. Specifically, teacher vaccination reduced secondary infections over 30 days to 91% and 97% of the average without vaccination in elementary and high schools, respectively. However, the reduction was slightly greater if the index case patient was a teacher, and in both settings, it reduced staff secondary incidence to about a third of its initial rate.
Weekly screening was projected to reduce secondary cases by a large degree compared with symptomatic isolation, classroom quarantine, or teacher vaccination, to an average of 0.3 to 0.8 cases with a 5-day schedule in elementary schools (varying by uptake of in-school prevention) and to 0.9 to 5.0 cases in high schools (Figure 1, right). The effect of weekly screening after a single introduced case was greatest for settings with low mitigation: Weekly screening reduced average projected secondary cases under classroom quarantine from 1.7 to 0.8 in elementary schools and from 23 to 5.0 in high schools.
Detection of School-Related Transmission
Across all scenarios, for elementary schools, we estimated that 73% of secondary cases would occur in students, 19% in families, and 8% in teachers and staff. For high schools, 78% would occur in students, 19% in families, and 4% in teachers and staff (Supplement Figures 2 and 3). Because children are less likely than adults to have symptoms, we projected that 14% of all secondary infections in elementary school communities and 15% in high school communities would be clinically symptomatic and therefore detectable without asymptomatic testing (Supplement Figure 4). With a more expansive definition of any symptoms, these percentages increased to 28% and 29%, respectively.
Stochastic Variation in Secondary Transmission
Rerunning the simulation 2000 times with each set of parameters, we observed considerable variability across possible outcomes (Figure 2). Elementary schools with high mitigation and classroom quarantine under a 5-day schedule had a 79% chance of 0 secondary cases over a 30-day period (Figure 2, top right). However, there was a 0.3% chance of more than 5 secondary cases. The chance of more than 5 secondary cases was higher when mitigation uptake was lower (Figure 2, top left and top center), reaching 52% of simulations with 0 secondary cases and 8.4% with 5 or more under low mitigation. In high schools with classroom quarantine, the probability of no secondary cases ranged from 18% to 51%, and the probability of more than 5 ranged from 12% to 61%, with long tails of outliers. However, with any interventions, the long tail of large outbreaks was substantially reduced.
Figure 2. Distribution of secondary transmissions when a single case is introduced.
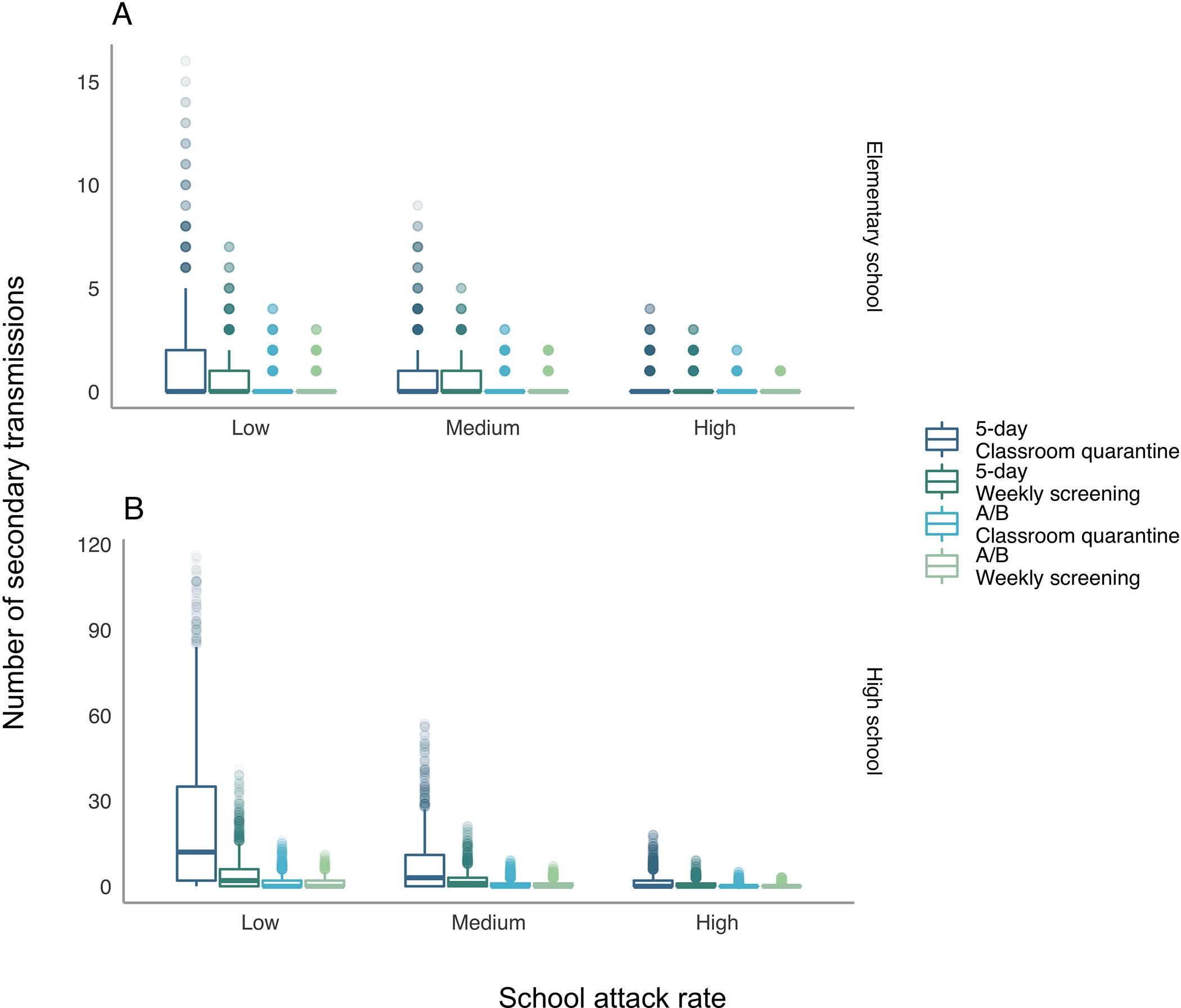
The figure displays the distribution of secondary transmissions (outside the index case patient’s household) when a single case is introduced into a school. The x-axis varies the level of in-school mitigation with “low” assuming minimal interventions and “high” assuming intensive interventions. Transmissions include both those directly from the index case and those from secondary and tertiary cases. Distributions are truncated at the 99.5th quantile (i.e., all outcomes occur with a probability ≥1/200). A/B = hybrid.
Transmissions Over the Course of the Semester
With any of the modeled scenarios (symptomatic isolation, classroom quarantine, teacher vaccination, and weekly screening), both 5-day and A/B schedules increased transmission compared with remote learning. This increase was greater when local incidence was higher, especially considering infection risk among staff (Figures 3 and 4). Assuming that additional measures, such as school closure, were not taken, Figures 3 and 4 show the values of local incidence at which our predefined threshold for “controlling transmission” was met. In elementary schools with medium mitigation under a 5-day schedule, all strategies with at least classroom quarantine met this threshold for all subgroups when local incidence decreased to or below 10 reported cases per 100 000 persons per day. With classroom quarantine, the control threshold for the full population was exceeded only at 100 cases per 100 000 persons per day (cumulative incidence, 16%; increment, 1.4 percentage points). However, among teachers, the control threshold was not met with moderate mitigation when local incidence was 25 cases or more per 100 000 persons per day (for example, total incidence of 7.0%; increment of 2.2 percentage points with classroom quarantine at 25 cases per 100 000), unless teachers were vaccinated. With both high mitigation and teacher vaccination, strategies met the control threshold among teachers at rates up to 50 cases per 100 000 persons per day.
Figure 3. Cumulative incidence over 8 weeks in elementary schools.
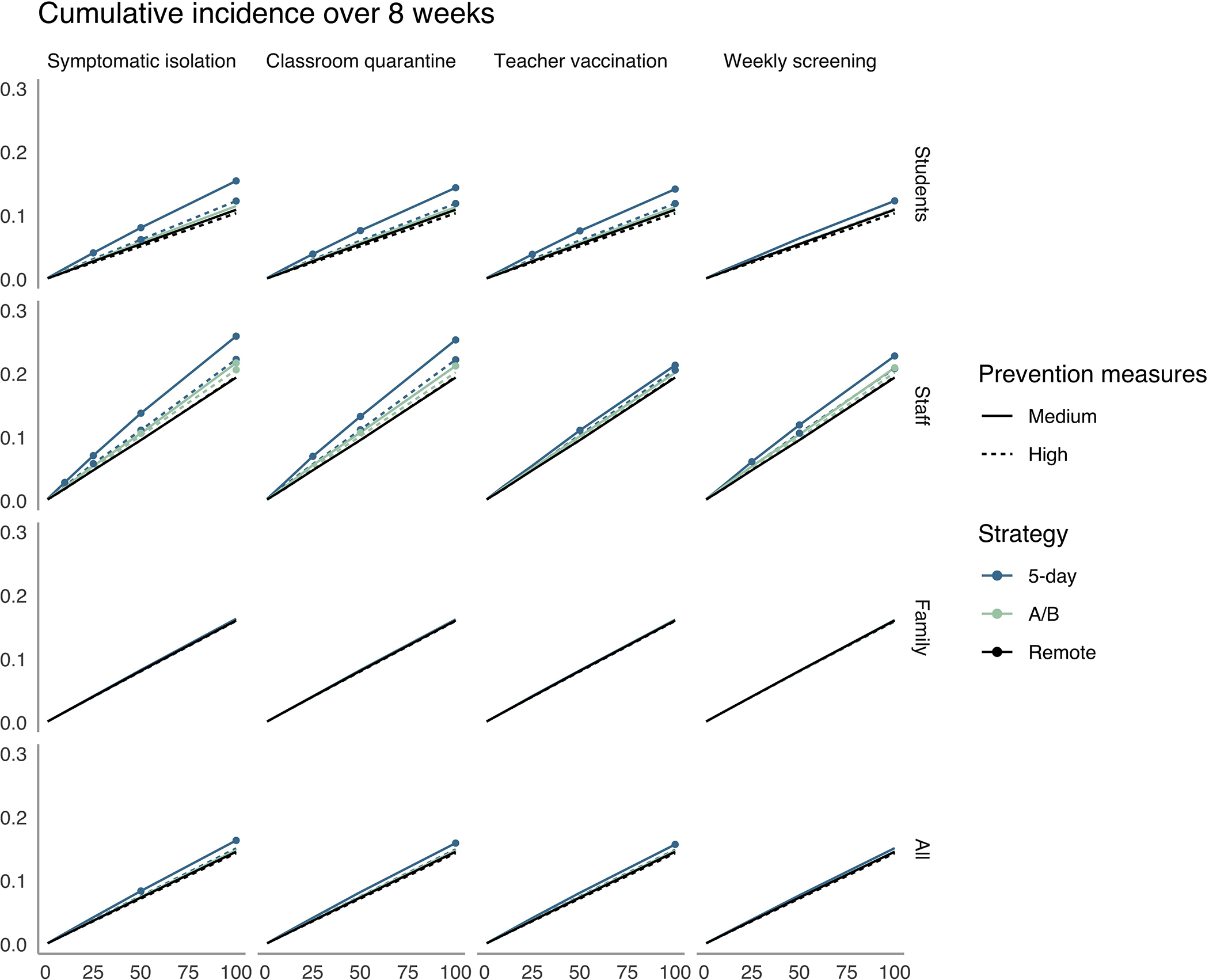
The x-axis shows the average daily local incidence per 100 000 persons in the population. The y-axis shows cumulative incidence over 8 weeks in the elementary school community. Columns denote isolation, quarantine, vaccination, and detection strategies, and rows show population subgroups. We mark a strategy with a point if it fails to meet the transmission control threshold for the subgroup (i.e., if there is a >1-point increase over remote learning in the percentage of the subgroup infected during 8 wk). A/B = hybrid.
Figure 4. Cumulative incidence over 8 weeks in high schools.
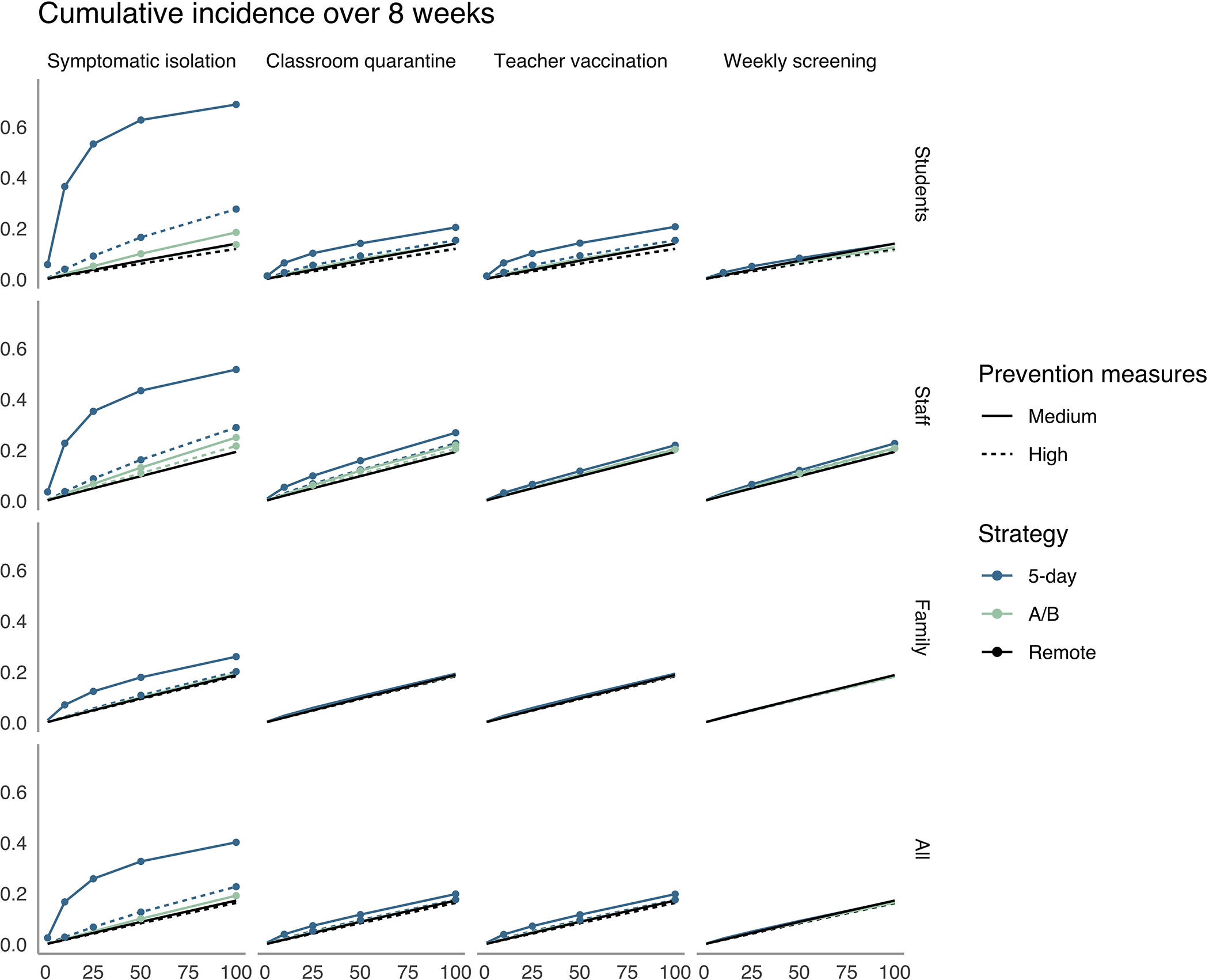
The x-axis shows the average daily local incidence per 100 000 persons in the population. The y-axis shows cumulative incidence over 8 wk in the high school community. Columns denote isolation, quarantine, vaccination, and detection strategies, and rows show population subgroups. We mark a strategy with a point if it fails to meet the transmission control threshold for the subgroup (i.e., if there is a >1-point increase over remote learning in the percentage of the group infected during 8 wk). A/B = hybrid.
In high schools, stronger mitigation or prevention strategies would be required to meet the control threshold. Under medium mitigation and local incidence of 100 cases per 100 000 persons per day, only weekly screening met this threshold for the full population with 5-day attendance. Under high mitigation, all strategies except symptomatic isolation met this threshold through 10 cases per 100 000, and exceeded it by only a small increment through 100 cases per 100 000 persons per day (maximum increment 1.3 percentage points for classroom quarantine at 100 cases per 100 000). For the teacher control threshold in high schools, high mitigation combined with teacher vaccination maintained the increase in teacher cumulative incidence below the control threshold at 50 cases per 100 000 persons per day.
Sensitivity Analyses
When we assumed that children were as infectious as adults, the average number of secondary cases in elementary schools over 30 days was 1.9 times higher than in the base case; if adolescents were half as susceptible as adults, secondary cases in high schools were reduced by a factor of 0.3 (Supplement Figures 1 and 5). When we repeated our analysis of elementary schools assuming that children and adults have equivalent variation in individual infectiousness, we found more instances of no onward transmission and a lower average number of secondary infections over 30 days, but also slightly larger outbreaks when they occurred (Supplement Figures 1 and 5). After a single introduction, all types of 2-day schedules (for example, Monday and Tuesday vs. Wednesday and Thursday) led to similar numbers of secondary infections overall, a similar chance of any secondary infection, and similar numbers of secondary infections among teachers and staff (Supplement Figures 1 and 5). Over the course of a quarter, the benefits of hybrid scheduling generally persisted across increased levels of out-of-school mixing (Supplement Figures 6 and 7). For student and caregiver secondary infections to be greater with an A/B model than a 5-day model in any scenario, at least 9 families needed to interact on each day out of school.
Discussion
Although in-person education poses some COVID-19 transmission risk, the results of this simulation model underscore that this risk can be offset with adequate precautions, particularly in elementary schools and when community transmission is well controlled. In elementary schools with adherence to masking and distancing, our results show that most cases introduced into a school would lead to little or no onward transmission. Nevertheless, if transmission occurs, it may be difficult to link to the school, and even modeled scenarios that commonly lead to no in-school transmission can occasionally generate larger outbreaks in a school community. The risk for this is substantially higher in high schools than elementary schools.
Although determining the exact risk for these low-probability but high-consequence events is difficult, local transmission determines the number of cases that enter a school, and therefore such rare but consequential outbreaks become increasingly likely when local incidence is high. The guidance from the Centers for Disease Control and Prevention about K-12 education highlights population-adjusted incidence as a core indicator (67), and our results provide additional evidence supporting use of this metric for school decisions in the absence of in-school screening or surveillance data. Nevertheless, although schools may decide that in-school transmission risk is too high above certain levels of local incidence, our results do not suggest that K-12 education with mitigation or modified schedules is likely to be a primary driver of sustained community transmission. In the event that the effective reproduction number (Rt) at the community level exceeds 1, it may be possible to lower this through targeted closure of high-risk venues and maintain an Rt below 1 while schools remain open.
Our results are compatible with global observations about school outbreaks, in which numerous well-studied index cases have produced no or minimal secondary cases; however, larger outbreaks have also been recorded, particularly in secondary schools (10, 68, 69). Teachers and staff tend to be overrepresented in school outbreaks relative to their presence in a school community. At the elementary level, they often represent a third or more of diagnosed secondary cases (7, 12). Because a substantial fraction of staff may be at high risk for complications, schools must undertake precautions to prevent transmission specifically among staff. Hospitals have similarly found patterns of staff-to-staff transmission and implemented such measures as reducing opportunities for communal food consumption (70).
We predict that most in-school transmission will occur in the classroom during sustained contact, and interventions that reduce classroom transmission can be highly effective, including distancing, masking, or reducing class sizes. Reduced density can be achieved through adding more staff and moving into previously unused spaces, allowing families to opt out of in-person learning while maintaining current staffing levels, prioritizing a subset of vulnerable students for limited in-person slots, or implementing hybrid scheduling. Some have raised concerns that a hybrid model could paradoxically increase SARS-CoV-2 transmission in schools by leading to greater out-of-cohort mixing on days when children are not physically in school. However, we find that the A/B schedule leads to fewer infections in the school community than a 5-day schedule, a result that persists even with an assumption of substantial out-of-school mixing between families.
Weekly screening for asymptomatic SARS-CoV-2 infection is particularly valuable for facilitating 5-day schedules in light of uncertainty in model parameters and outcomes. There is still debate surrounding the relative susceptibility and transmissibility of children compared with adults; overdispersion of infectiousness; and, in particular, the degree to which asymptomatic children transmit infection—all of which have substantial effects on transmission in a school setting. It may also be difficult to ascertain local behavioral parameters, such as adherence to masking, distancing, and quarantine protocols, which can vary across settings and over time. Coupled with stochastic uncertainty in outbreak size and a high proportion of subclinical illnesses in school populations, this variability may make it difficult to detect transmission when it occurs. The low probability of clinical disease for infected children means that transmission chains in schools are likely to be only partially observed and linked cases may be mistakenly classified as isolated introductions. Regular screening can both improve these data and prevent transmission through early detection; however, at a minimum, schools should be on alert for signs that an outbreak is brewing and consider screening in response to the detection of cases without a clearly identified source. Other factors not considered in this work include changes in parent behavior and increased out-of-home work when children return to school, particularly for mothers and single parents, as well as changes in staff social interactions outside school (71, 72).
In addition, new variants of concern have recently been identified that are more transmissible than the strains that have previously dominated (73, 74). One variant, B.1.1.7, has been linked to a large outbreak in a primary school in the Netherlands and triggered a new wave of school closures in Europe (19, 75). In areas where these strains dominate in the United States, classroom-based efforts at infection prevention, such as masking and distancing, may be less effective at suppressing attack rates. For instance, schools that have achieved attack rates commensurate with medium uptake of mitigation may find that the same measures will result in attack rates reflecting low uptake. This shift highlights the urgent need for data and underscores the added value of routine asymptomatic screening.
Tradeoffs are inevitable between school disruption, risk for in-school transmission, and resources required to implement reopening strategies. We offer a quantitative perspective on the way that in-school transmissions are likely to occur, as well as the effect that proposed protocols could have on that transmission risk. We emphasize that, particularly among young children, schools seem to be “mirrors” of local transmission rather than “amplifiers” or “brakes.” Thus, a reliable way to ensure a low infection risk in schools is to reduce infectious introductions by keeping local incidence low. However, even when introductions occur, high adherence to in-school prevention measures, complemented by regular asymptomatic testing and teacher vaccination, can also permit return to in-person education with controlled risk for COVID-19 transmission in schools. Local, state, and federal agencies should prioritize these effective interventions that permit the benefits of in-person education while protecting the safety of both students and educators.
Supplementary Material
Appendix Figure 1. Model diagram. The model includes 3 primary domains (households, schools, and out-of-school social or childcare mixing) and incorporates a range of interventions to prevent or reduce transmission.
Acknowledgments
Financial Support: By grant NU38OT000297–02 from the Centers for Disease Control and Prevention though the Council of State and Territorial Epidemiologists (Ms. Bilinski and Dr. Salomon), grants R37AI058736–16S1 (Dr. Ciaranello) and K01AI141576 (Dr. Fitzgerald) from the National Institute of Allergy and Infectious Diseases, grant 3R37DA01561217S1 from the National Institute on Drug Abuse (Dr. Salomon), and an unrestricted gift from Facebook (Mr. Giardina, Ms. Bilinski, and Dr. Salomon).
Footnotes
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M21–0600.
Reproducible Research Statement: Study protocol: NA Statistical code: Model code is publicly available as an R package at https://github.com/abilinski/BackToSchool2. Replication code is also available on GitHub. Data set: Simulated data is available on GitHub.
Contributor Information
Alyssa Bilinski, Interfaculty Initiative in Health Policy, Harvard Graduate School of Arts and Sciences, Cambridge, Massachusetts.
Joshua A. Salomon, Center for Health Policy and Center for Primary Care and Outcomes Research, Stanford University School of Medicine, Stanford, California.
John Giardina, Interfaculty Initiative in Health Policy, Harvard Graduate School of Arts and Sciences, Cambridge, and Center for Health Decision Science, Harvard T.H. Chan School of Public Health, Boston, Massachusetts.
Andrea Ciaranello, Medical Practice Evaluation Center, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts.
Meagan C. Fitzpatrick, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland.
References
- 1.Map: coronavirus and school closures. Education Week. 6 March 2020. Accessed at www.edweek.org/ew/section/multimedia/map-coronavirus-and-school-closures.html on 11 June 2020.
- 2.K-12 school opening tracker. Burbio. 2020. Accessed at https://cai.burbio.com/school-opening-tracker on 25 November 2020.
- 3.2020–2021 dashboard. Boston Public Schools. 2021. Accessed at http://datastudio.google.com/reporting/77e82eef-349a-4f00-bd50-0824cd0df332/page/1vChB?feature=opengraph on 21 April 2021.
- 4.Over Shapiro E. 50,000 N.Y.C. public school students will return to classrooms, including in middle and high school. New York Times. 12 April 2021. Accessed at www.nytimes.com/2021/04/12/nyregion/nyc-public-schools-students.html on 21 April 2021.
- 5.Shapiro E How de Blasio backed himself into a corner on closing schools. New York Times. 24 November 2020. Accessed at www.nytimes.com/2020/11/24/nyregion/deblasio-school-reopening.html on 25 November 2020.
- 6.Combating COVID-19. President-Elect Joe Biden. 2020. Accessed at https://buildbackbetter.gov/priorities/covid-19 on 25 November 2020. [Google Scholar]
- 7.Macartney K, Quinn HE, Pillsbury AJ, et al. ; NSW COVID-19 Schools Study Team. Transmission of SARS-CoV-2 in Australian educational settings: a prospective cohort study. Lancet Child Adolesc Health. 2020;4:807–816. [PMID: 32758454] doi: 10.1016/S2352-4642(20)30251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontanet A, Grant R, Tondeur L, et al. SARS-CoV-2 infection in primary schools in northern France: a retrospective cohort study in an area of high transmission. medRxiv. Preprint posted online 29 June 2020. doi: 10.1101/2020.06.25.20140178 [DOI] [Google Scholar]
- 9.Yung CF, Kam KQ, Nadua KD, et al. Novel coronavirus 2019 transmission risk in educational settings. Clin Infect Dis. 2021;72:1055–1058. [PMID: 32584975] doi: 10.1093/cid/ciaa794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein-Zamir C, Abramson N, Shoob H, et al. A large COVID-19 outbreak in a high school 10 days after schools’ reopening, Israel, May 2020. Euro Surveill. 2020;25. [PMID: 32720636] doi: 10.2807/1560-7917.ES.2020.25.29.2001352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iowa COVID-19 tracker. 2020. Accessed at https://iowacovid19tracker.org on 25 November 2020.
- 12.Torres JP, Piñera C, De La Maza V, et al. SARS-CoV-2 antibody prevalence in blood in a large school community subject to a Covid-19 outbreak: a cross-sectional study. Clin Infect Dis. 2020. [PMID: 32649743] doi: 10.1093/cid/ciaa955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auger KA, Shah SS, Richardson T, et al. Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA. 2020;324:859–870. [PMID: 32745200] doi: 10.1001/jama.2020.14348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haug N, Geyrhofer L, Londei A, et al. Ranking the effectiveness of worldwide COVID-19 government interventions. Nat Hum Behav. 2020;4:1303–1312. [PMID: 33199859] doi: 10.1038/s41562-020-01009-0 [DOI] [PubMed] [Google Scholar]
- 15.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145. [PMID: 32179660] doi: 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 16.Reuters Staff. Reopening schools in Denmark did not worsen outbreak, data shows. Reuters. 28 May 2020. Accessed at www.reuters.com/article/us-health-coronavirus-denmark-reopening-idUSKBN2341N7 on 11 June 2020.
- 17.Pancevski B, Dandanell N. Is it safe to reopen schools? These countries say yes. Wall Street Journal. 31 May 2020. Accessed at www.wsj.com/articles/is-it-safe-to-reopen-schools-these-countries-say-yes-11590928949 on 11 June 2020.
- 18.Vogel G, Couzin-Frankel J. As COVID-19 soars in many communities, schools attempt to find ways through the crisis. Science. 2020. doi: 10.1126/science.abf7779 [DOI] [Google Scholar]
- 19.Hall B, Staton B, Chaffin J, et al. European capitals follow UK with school closures as virus surges. Financial Times. 7 January 2021. Accessed at www.ft.com/content/8121ca0a-4d96-4cf5-b5df-a73adc16a606 on 22 January 2021.
- 20.Rapaport A, Saavedra A, Silver D, et al. Surveys show things are better for students than they were in the spring—or do they? Brookings Institution. 18 November 2020. Accessed at www.brookings.edu/blog/brown-center-chalkboard/2020/11/18/surveys-show-things-are-better-for-students-than-they-were-in-the-spring-or-do-they on 20 November 2020.
- 21.Gaudiano N Missing: millions of students. Politico. 26 October 2020. Accessed at https://politi.co/3dVrNxg on 25 November 2020.
- 22.Leeb RT, Bitsko RH, Radhakrishnan L, et al. Mental health–related emergency department visits among children aged <18 years during the COVID-19 pandemic — United States, January 1–October 17, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1675–1680. [PMID: 33180751] doi: 10.15585/mmwr.mm6945a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hess AJ. Widespread school closures mean 30 million kids might go without meals. CNBC. 14 March 2020. Accessed at www.cnbc.com/2020/03/14/widespread-school-closures-mean-30-million-kids-might-go-without-meals.html on 1 August 2020.
- 24.Baron EJ, Goldstein EG, Wallace CT. Suffering in silence: how COVID-19 school closures inhibit the reporting of child maltreatment. SSRN. Preprint posted online 17 May 2020. doi: 10.2139/ssrn.3601399 [DOI] [PMC free article] [PubMed]
- 25.Bueno C Bricks and mortar vs. computers and modems: the impacts of enrollment in K-12 virtual schools. SSRN. Preprint posted online 5 July 2020. doi: 10.2139/ssrn.3642969 [DOI]
- 26.Lempel H, Hammond RA, Epstein JM. Costs of school closure. Brookings Institution. 30 September 2009. Accessed at www.brookings.edu/wp-content/uploads/2016/06/0930_school_closure_presentation.pdf on 1 August 2020.
- 27.Soland J, Kuhfeld M, Tarasawa B, et al. The impact of COVID-19 on student achievement and what it may mean for educators. Brookings Institution. 27 May 2020. Accessed at www.brookings.edu/blog/brown-center-chalkboard/2020/05/27/the-impact-of-covid-19-on-student-achievement-and-what-it-may-mean-for-educators on 1 August 2020.
- 28.Cohen JA, Mistry D, Kerr CC, et al. Schools are not islands: balancing COVID-19 risk and educational benefits using structural and temporal countermeasures. medRxiv. Preprint posted online 10 September 2020. doi: 10.1101/2020.09.08.20190942 [DOI] [Google Scholar]
- 29.España G, Cavany S, Oidtman R, et al. Impacts of K-12 school reopening on the COVID-19 epidemic in Indiana, USA. medRxiv. Preprint posted online 14 September 2020. doi: 10.1101/2020.08.22.20179960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Head JR, Andrejko KL, Cheng Q, et al. The effect of school closures and reopening strategies on COVID-19 infection dynamics in the San Francisco Bay Area: a cross-sectional survey and modeling analysis. medRxiv. Preprint posted online 7 August 2020. doi: 10.1101/2020.08.06.20169797 [DOI] [Google Scholar]
- 31.Bershteyn A, Kim HY, McGillen J, et al. Which policies most effectively reduce SARS-CoV-2 transmission in schools? medRxiv. Preprint posted online 27 November 2020. doi: 10.1101/2020.11.24.20237305 [DOI] [Google Scholar]
- 32.McGee RS, Homburger JR, Williams HE, et al. Model-driven mitigation measures for reopening schools during the COVID-19 pandemic. medRxiv. Preprint posted online 6 February 2021. doi: 10.1101/2021.01.22.21250282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park YJ, Choe YJ, Park O, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26:2465–2468. [PMID: 32673193] doi: 10.3201/eid2610.201315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. COVID-19 and your health. 2020. Accessed at www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/quarantine.html on 4 January 2021. [PubMed]
- 35.National Center for Education Statistics. Number and percentage distribution of public elementary and secondary schools and enrollment, by level, type, and enrollment size of school: 2015–16, 2016–17, and 2017–18. Digest of Education Statistics. 2019. Accessed at https://nces.ed.gov/programs/digest/d19/tables/dt19_216.40.asp?current=yes on 12 December 2020. [Google Scholar]
- 36.Byambasuren O, Cardona M, Bell K, et al. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. J Assoc Med Microbiol Infect Dis Can. 2020;5:223–34. doi: 10.3138/jammi-2020-0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han MS, Choi EH, Chang SH, et al. Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the Republic of Korea. JAMA Pediatr. 2021;175:73–80. [PMID: 32857112] doi: 10.1001/jamapediatrics.2020.3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madewell ZJ, Yang Y, Longini IM Jr, et al. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open. 2020;3:e2031756. [PMID: 33315116] doi: 10.1001/jamanetworkopen.2020.31756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fung HF, Martinez L, Alarid-Escudero F, et al. The household secondary attack rate of SARS-CoV-2: a rapid review. Clin Infect Dis. 2020. [PMID: 33045075] doi: 10.1093/cid/ciaa1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerr CC, Stuart RM, Mistry D, et al. Covasim: an agent-based model of COVID-19 dynamics and interventions. medRxiv. Preprint posted online 1 April 2021. doi: 10.1101/2020.05.10.20097469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Endo A, Abbott S, Kucharski AJ, et al. Estimating the overdispersion in COVID-19 transmission using outbreak sizes outside China. Wellcome Open Res. 2020;5:67. doi: 10.12688/wellcomeopenres.15842.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. [PMID: 32150748] doi: 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. [PMID: 31995857] doi: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gatto M, Bertuzzo E, Mari L, et al. Spread and dynamics of the COVID-19 epidemic in Italy: effects of emergency containment measures. Proc Natl Acad Sci U S A. 2020;117:10484–10491. [PMID: 32327608] doi: 10.1073/pnas.2004978117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. [PMID: 32296168] doi: 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 46.He D, Zhao S, Lin Q, et al. The relative transmissibility of asymptomatic COVID-19 infections among close contacts. Int J Infect Dis. 2020;94:145–147. [PMID: 32315808] doi: 10.1016/j.ijid.2020.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Firth JA, Hellewell J, Klepac P, et al. Combining fine-scale social contact data with epidemic modelling reveals interactions between contact tracing, quarantine, testing and physical distancing for controlling COVID-19. medRxiv. Preprint posted online 2 July 2020. doi: 10.1101/2020.05.26.20113720 [DOI] [Google Scholar]
- 48.Brenan M Willingness to get COVID-19 vaccine ticks up to 63% in U.S. Gallup. 8 December 2020. Accessed at https://news.gallup.com/poll/327425/willingness-covid-vaccine-ticks.aspx on 4 January 2021.
- 49.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. [PMID: 33301246] doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atkeson A, Droste M, Mina MJ, et al. Economic benefits of COVID-19 screening tests with a vaccine rollout. medRxiv. Preprint posted online 5 March 2021. doi: 10.1101/2021.03.03.21252815 [DOI] [Google Scholar]
- 51.Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7. [PMID: 33219112] doi: 10.1126/sciadv.abd5393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cevik M, Tate M, Lloyd O, et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2:e13–e22. [PMID: 33521734] doi: 10.1016/S2666-5247(20)30172-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wyllie AL, Fournier J, Casanovas-Massana A, et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2 [Letter]. N Engl J Med. 2020;383:1283–1286. [PMID: 32857487] doi: 10.1056/NEJMc2016359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kojima N, Turner F, Slepnev V, et al. Self-collected oral fluid and nasal swab specimens demonstrate comparable sensitivity to clinician-collected nasopharyngeal swab specimens for the detection of SARS-CoV-2. Clin Infect Dis. 2020. [PMID: 33075138] doi: 10.1093/cid/ciaa1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orscheln RC, Newland JG, Rosen DA. Practical school algorithms for symptomatic or SARS-CoV-2-exposed students are essential for returning children to in-person learning. J Pediatr. 2021;229:275–277. [PMID: 32980377] doi: 10.1016/j.jpeds.2020.09.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Massachusetts Chapter of the American Academy of Pediatrics. COVID & kids. Accessed at https://mcaap.org/2018/wp-content/uploads/new-handout-copy.pages-FINAL-1.pdf on 19 April 2021.
- 57.Will M School workers may get early shot at COVID-19 vaccine. Will they take it? Education Week. 21 December 2020. Accessed at www.edweek.org/leadership/school-workers-may-get-early-shot-at-covid-19-vaccine-will-they-take-it/2020/12 on 4 January 2021.
- 58.Wheaton WD. U.S. Synthetic Population 2010 Version 1.0 Quick Start Guide. RTI International; 2014.
- 59.Cheng HY, Jian SW, Liu DP, et al. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180:1156–1163. [PMID: 32356867] doi: 10.1001/jamainternmed.2020.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clapp PW, Sickbert-Bennett EE, Samet JM, et al. Evaluation of cloth masks and modified procedure masks as personal protective equipment for the public during the COVID-19 pandemic. JAMA Intern Med. 2021;181:463–469. [PMID: 33300948] doi: 10.1001/jamainternmed.2020.8168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. [PMID: 32497510] doi: 10.1016/S0140-6736(20)31142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175:143–156. [PMID: 32975552] doi: 10.1001/jamapediatrics.2020.4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dattner I, Goldberg Y, Katriel G, et al. The role of children in the spread of COVID-19: using household data from Bnei Brak, Israel, to estimate the relative susceptibility and infectivity of children. PLOS Computational Biology. 2021. February 11;17(2):e1008559. [PMID: 33571188] doi: 10.1371/journal.pcbi.1008559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fontanet A, Tondeur L, Madec Y, et al. Cluster of COVID-19 in northern France: a retrospective closed cohort study. medRxiv. Preprint posted online 23 April 2020. doi: 10.1101/2020.04.18.20071134 [DOI] [Google Scholar]
- 65.Gu Y COVID-19 Projections Using Machine Learning. Accessed at https://covid19-projections.com on 21 April 2021.
- 66.Global Epidemics. Schools and the path to zero. Accessed at https://globalepidemics.org/2020/12/18/schools-and-the-path-to-zero on 20 January 2021.
- 67.Centers for Disease Control and Prevention. Indicators for Dynamic School Decision-Making. Accessed at www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/indicators.html on 25 November 2020.
- 68.Link-Gelles R, DellaGrotta AL, Molina C, et al. Limited secondary transmission of SARS-CoV-2 in child care programs — Rhode Island, June 1–July 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1170–1172. [PMID: 32853185] doi: 10.15585/mmwr.mm6934e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ladhani S, Ahmad S, Garstang J, Brent AJ, Brent B. Prospective active national surveillance of preschools and primary schools for SARS-CoV-2 infection and transmission in England, June 2020.:23. Public Health England. Accessed at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/914700/sKIDs_Phase1Report_01sep2020.pdf. [DOI] [PMC free article] [PubMed]
- 70.Statement for media regarding COVID-19 cluster. Brigham and Women’s Hospital. 16 October 2020. Accessed at www.brighamandwomens.org/about-bwh/newsroom/press-releases-detail?id=3684 on 25 November 2020.
- 71.Beauregard PL, Connolly M, Haeck C, et al. Primary school reopenings and parental work. Research Group on Human Capital, University of Quebec in Montreal’s School of Management. 2020. Accessed at https://ideas.repec.org/p/grc/wpaper/20-06.html on 16 March 2021.
- 72.Collins C, Ruppanner L, Landivar LC, et al. The gendered consequences of a weak infrastructure of care: school reopening plans and parents’ employment during the COVID-19 pandemic. Gend Soc. 2021;35:180–93. doi: 10.1177/08912432211001300 [DOI] [Google Scholar]
- 73.Volz E, Mishra S, Chand M, et al. Transmission of SARS-CoV-2 lineage B.1.1.7 in England: insights from linking epidemiological and genetic data. medRxiv. Preprint posted online 4 January 2021. doi: 10.1101/2020.12.30.20249034 [DOI] [Google Scholar]
- 74.Walensky RP, Walke HT, Fauci AS. SARS-CoV-2 variants of concern in the United States—challenges and opportunities. JAMA. 2021;325:1037–1038. [PMID: 33595644] doi: 10.1001/jama.2021.2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vogel G School risk calculations scrambled by fast-spreading virus strains. Science. 2021. doi: 10.1126/science.abg6030 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Figure 1. Model diagram. The model includes 3 primary domains (households, schools, and out-of-school social or childcare mixing) and incorporates a range of interventions to prevent or reduce transmission.


