Figure 1.
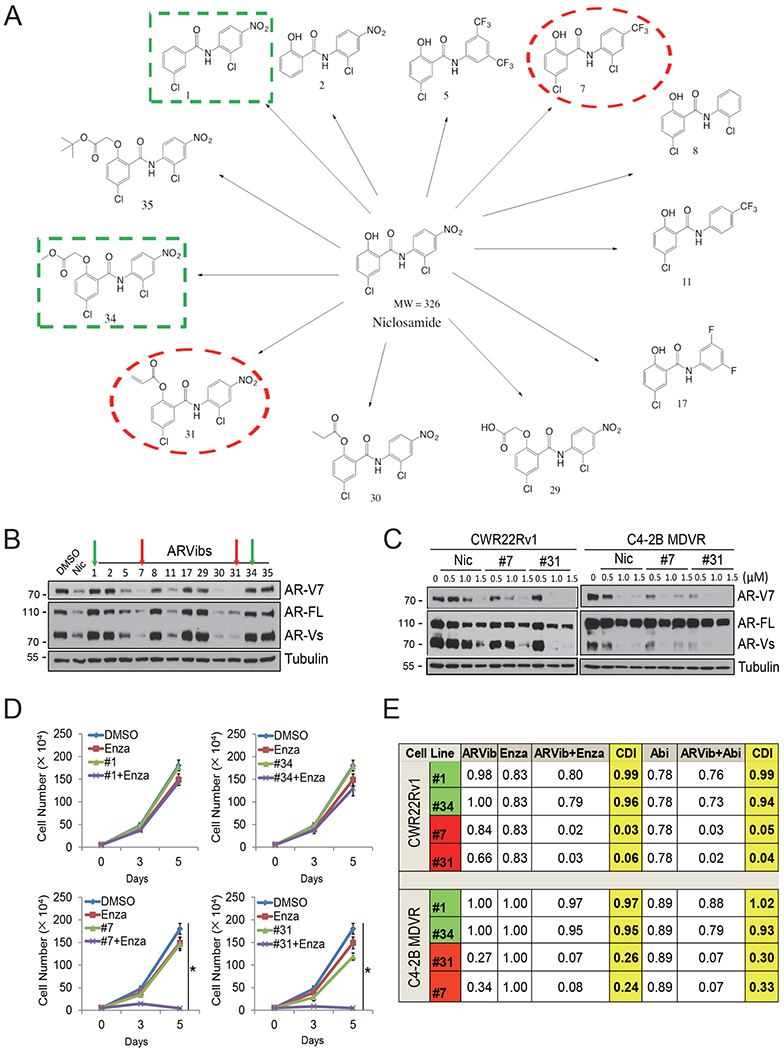
Synthesis of niclosamide analogs and identification of potent inhibitors of AR/AR variants. A. The chemical structure of niclosamide and newly synthesized ARVibs. The red box indicates the structures of #7 and #31, the green box indicates chemical structures of #1 and #34. B. CWR22Rv1 cells were treated with 1 ¼M ARVib for 16 hours and whole cell lysates were collected and subjected to western blot. C. C4-2B MDVR and CWR22Rv1 cells were treated with different doses of ARVibs for 16 hours and whole cell lysates were collected and subjected to western blot. D. CWR22Rv1 cells were treated with enzalutamide with or without ARVibs (#1, #34, #7 and #31), total cell numbers were determined at 0, 3, 5 days. E. The CDI of enzalutamide or abiraterone with ARVibs in CWR22Rv1 and C4-2B MDVR cells were calculated. * p˂0.05. Results are the mean of three independent experiments (±S.D.). AR-FL: full-length AR, AR-Vs: AR-Variants, Enza: enzalutamide, Abi: abiraterone, Nic: niclosamide, CDI: coefficient of drug interaction.
