Fig. 2.
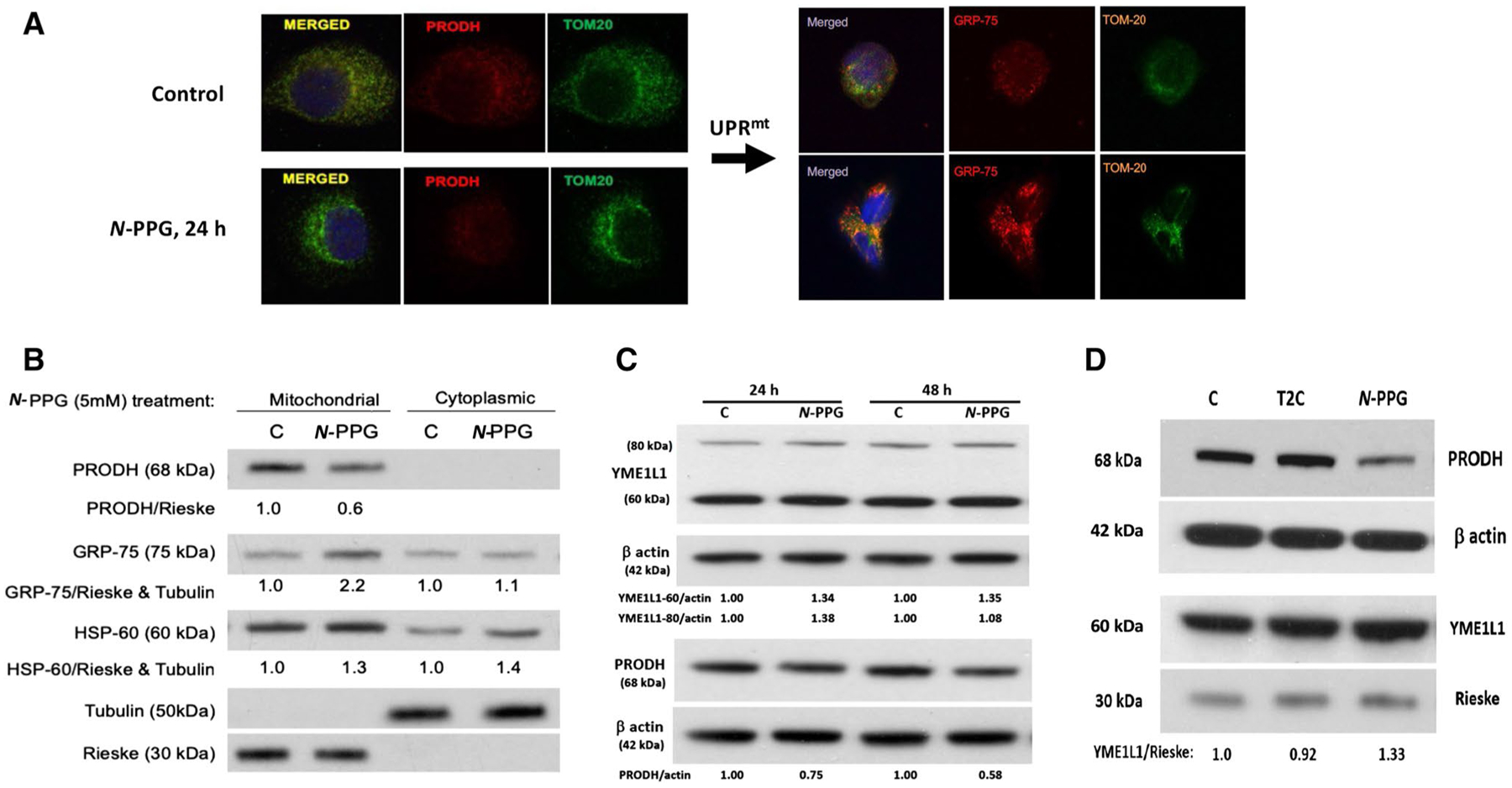
Treatment of human breast cancer cells ZR-75-1 with N-PPG, but not with T2C, activates mitochondrial PRODH degradation and increases mitochondrial expression of chaperone proteins (GRP-75, HSP-60) and the inner mitochondrial protease, YME1L1, consistent with UPRmt induction. A Confocal imaging of ZR-75-1 cells (63X oil immersion magnification) treated with vehicle (control) or N-PPG (5 mM × 24 h), PRODH (red, left panel) or GRP-75 (red, right panel), or TOM20 (green, left and right panels) stained with mouse primaries and detected by fluorochrome-conjugated secondary antibodies. Merged images confirm mitochondrial co-localization. B Western blot of mitochondrial vs. cytoplasmic expression of PRODH, GRP-75, HSP-60, tubulin, and Rieske proteins 24 h after cell culture treatment of ZR-75-1 cells with N-PPG (5 mM) or drug vehicle (C). C. Western blots comparing PRODH downregulation with 60 kDa YME1L1 upregulation in whole cell lysates of ZR-75-1 cells after 24 h and 48 h exposures to N-PPG (5 mM) or vehicle (C). Upon import into mitochondria, cleavage of the mitochondrial targeting sequence from newly induced 80 kDa cytosolic YME1L1 produces the mitochondrial membrane-localizing and longer lasting 60 kDa YME1L1 (Hartmann 2016). D Western blots of ZR-75-1 whole cell lysates comparing expression of PRODH (68 kDa), YME1L1 (60 kDa), β-actin (42 kDa), and mitochondrial Rieske (30 kDa) after 24 h cell culture treatment with vehicle (C), N-PPG (5 mM), or T2C (5 mM)
