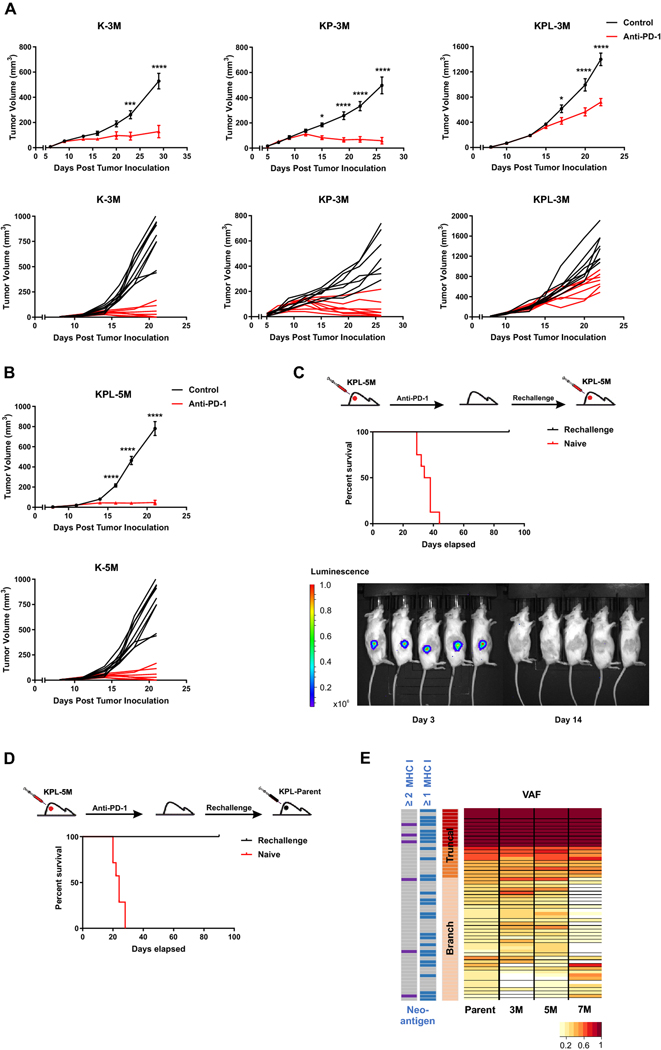
Fig. 4.
High TMB results in increased efficacy of anti-PD-1 therapy. a After SC tumor inoculation [K-3M (2 × 106) cells in 129-E mice; KP-3M (2 × 106) cells in FVB mice; KPL-3M (1.5 × 105) cells in FVB mice], mice bearing < 50mm3 tumors (∼ day 7) were treated with (i) isotype control, (ii) anti-PD-1 (200 μg/dose 3 times weekly for 4 doses), and tumor growth was measured with caliper. Results are representatives of at least two biological replicates of 6–10 mice per group. b Same experimental design as a except that KPL-5M (3 × 105) cells were utilized for SC tumor inoculation. c FVB naïve mice and mice that previously eradicated KPL-5M tumors in response to PD-1 blockade were inoculated SC with KPL-5M (3 × 105) and tumor growth was measured with bioluminescence imaging on day 3 and day 14. Survival curve is presented. Data is representatives of two biological replicates of 5–6 mice per group. d FVB naïve mice and FVB mice that previously eradicated KPL-5M tumors in response to PD-1 blockade were inoculated SC with KPL-Parent (2 × 105). Survival curve is presented. Data is representatives of two biological replicates of 5–6 mice per group. e Representation of the frequency of the mutations in the KPL-Parent tumors shared by KPL-3M, KPL-5M, or KPL-7M. The predicted neoantigens are presented based on MHC-I binding avidity by at least one or two MHC-I alleles (two left columns). P values were determined by two-way ANOVA with Tukey post-test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001