Fig. 1.
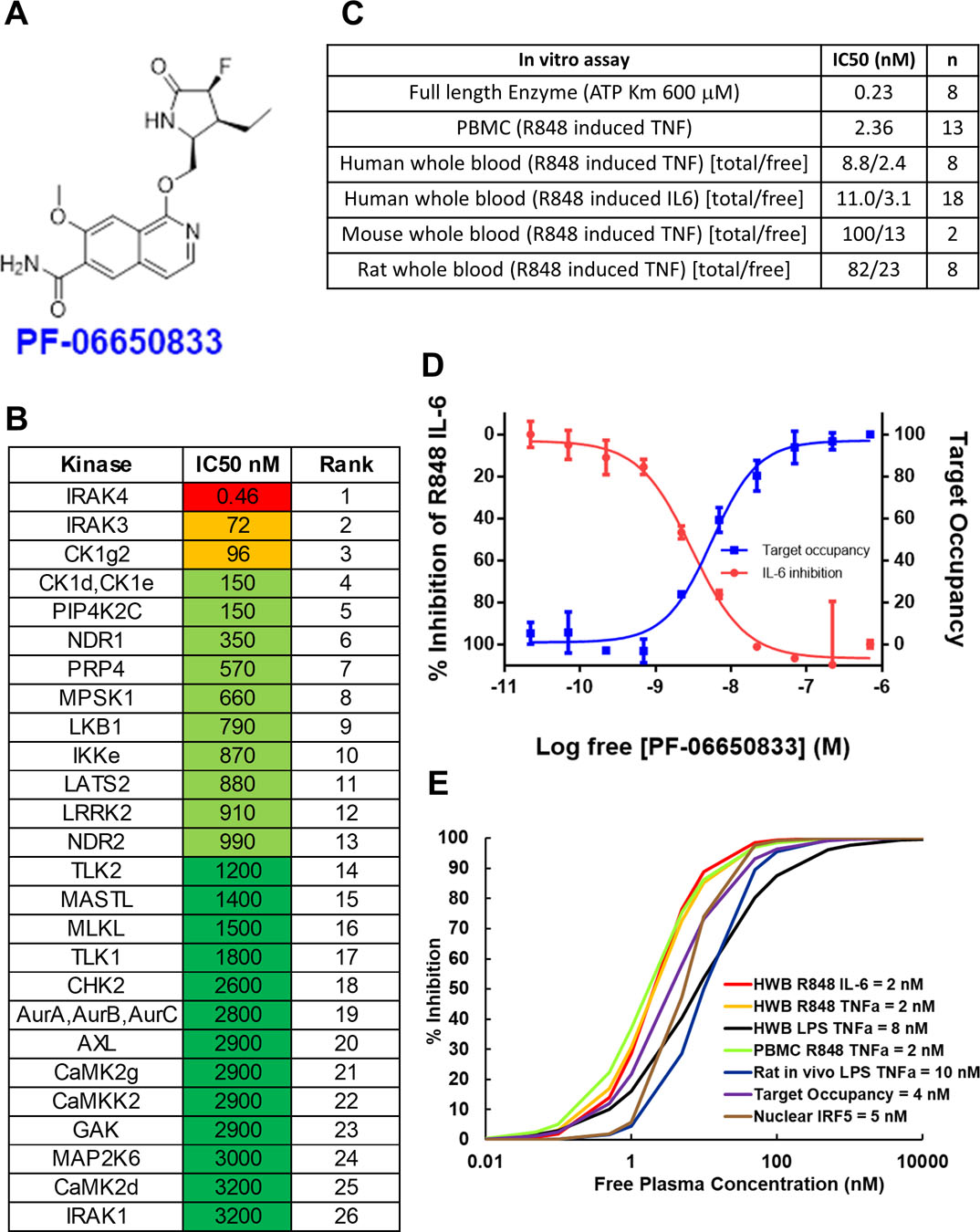
Pharmacological properties of PF-06650833. (A) Structure of PF-06650833. (B) Selectivity of PF-06650833 in ActivX ATP occupancy assay in THP1 lysates. The top 26 out of 200+ kinases are shown. IC50s were determined by a 5-point dose response curve (full data set in table S1) (C) Potency of PF-06650833 in enzyme and cell-based assays. IC50 values for enzyme and PBMC assays are a single value, whereas whole blood (wb) assays for human, rat and mouse are denoted as total concentration of compound over the non-protein bound (free=f) concentration of compound. (wb IC50*[fu/(B/P)] = IC50 free, where fu = 0.22, B/P = Blood/plasma ratio = 0.91). (D) Demonstration of the relationship between free drug concentration, IRAK4 ATP binding site occupancy, and inhibition of downstream pharmacology by PF-06650833 in a sample of human SLE whole blood. (E) EC50 data for each pre-clinical assay were used to determine that 100 nM was the target free compound concentration was required to maintain greater than 90% IRAK4 inhibition across species and assays.
