FIGURE 12.
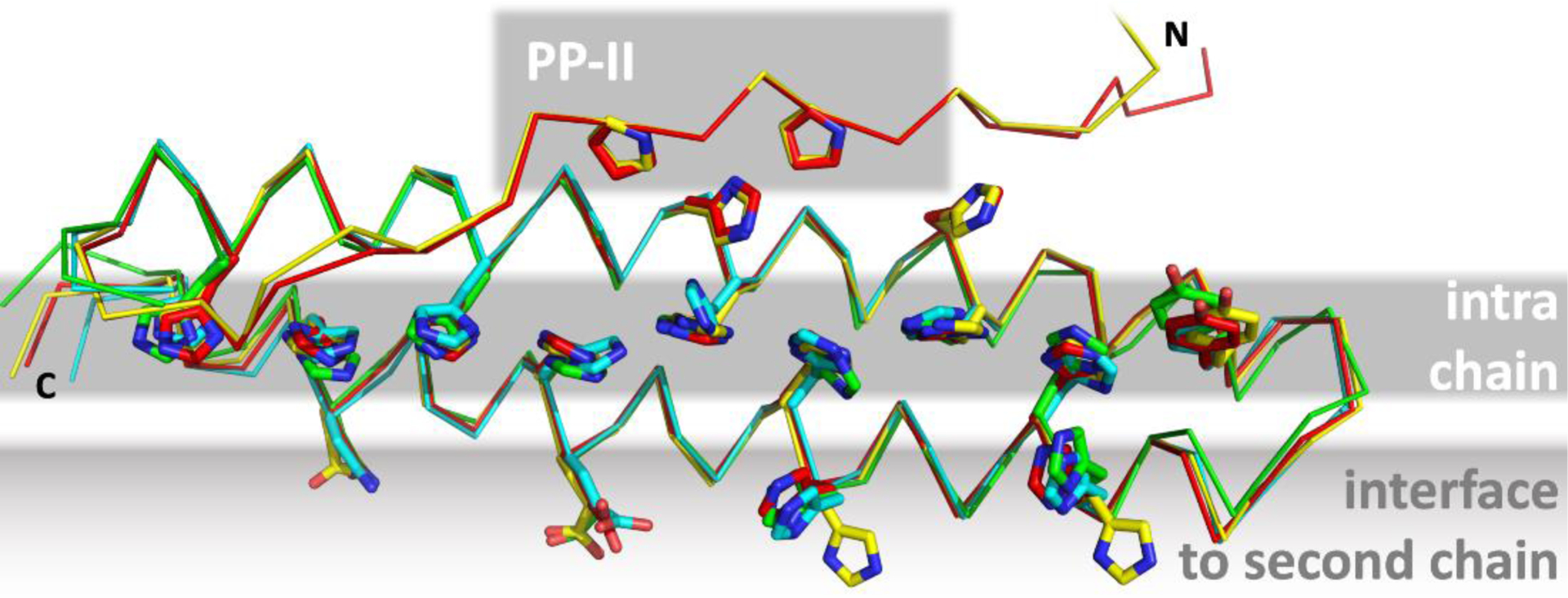
Single chain of the Tuna (T1087) crystal structure (yellow) superimposed with exemplary predictions (red: AlphaFold2; cyan: FEIG-S, green: BAKER-ROBETTA). Tuna forms two coiled-coil interfaces, one intra-chain and one for homo-dimerization with another chain. Although only the monomer was a prediction target in CASP 14, several residues of the inter-chain interface were predicted in the correct rotamer by the top-ranking groups and servers. The N-terminal extension containing a polyproline-II helix is only shown for AlphaFold2, which predicted it most precisely in conformation and position.
