Fig. 6.
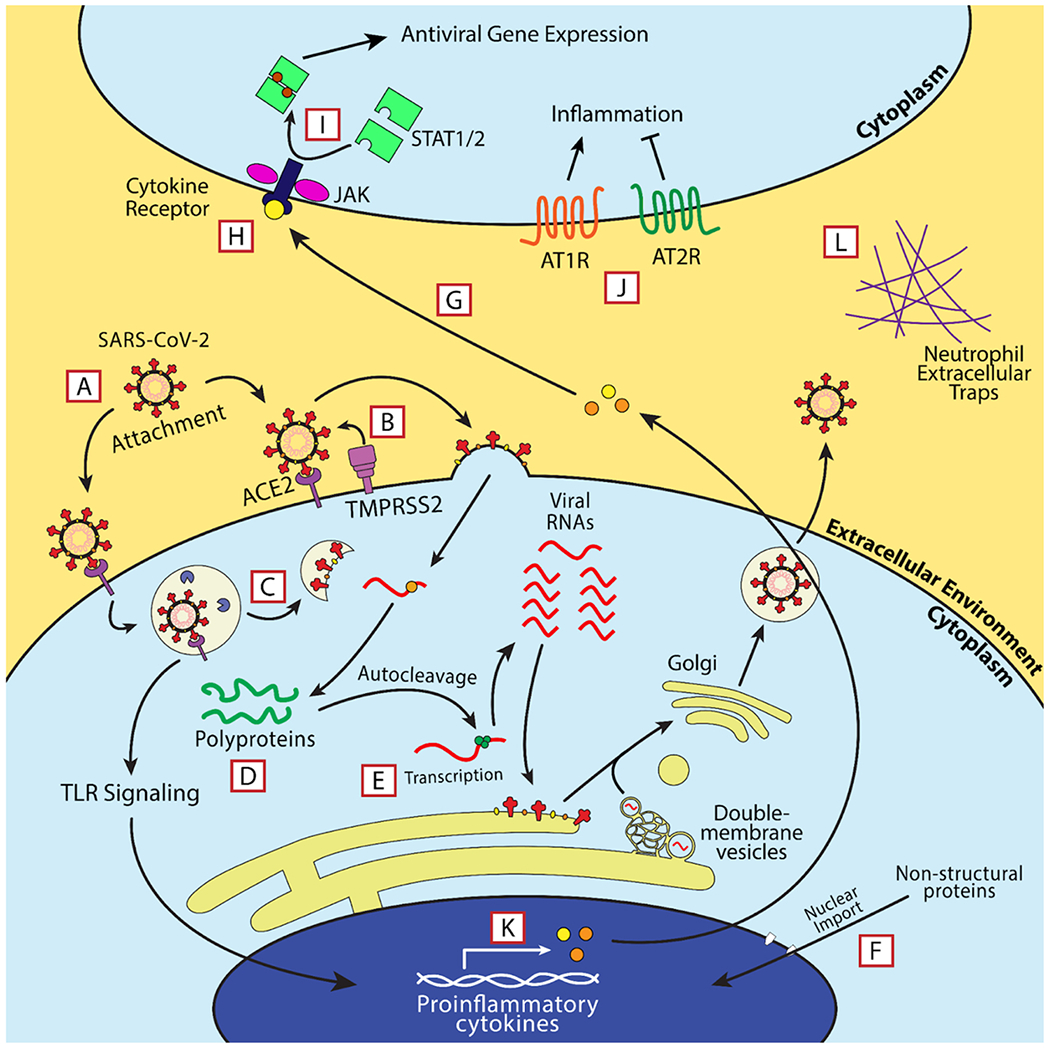
Ongoing or published clinical trials of therapeutics used during different phases of COVID-19. Each letter in a red box describes the step in viral replication or the immune response that at least one investigated drug targets. Each letter’s corresponding treatments are listed below. A - Etesevimab, Bamlanivimab, Convalescent sera. B – Proxalutamide. C – Hydroxychloroquine. D - Lopinavir/Ritonavir. E - Remdesivir, Favipiravir, Molnupiravir. F – Ivermectin. G – Tocilizumab. H - Peginterferon – λ. I - Baricitinib, Ruxolitinib. J - Losartan, Compound 21 (C21). K - Dexamethasone, Methylprednisolone. L - Dornase alfa.
