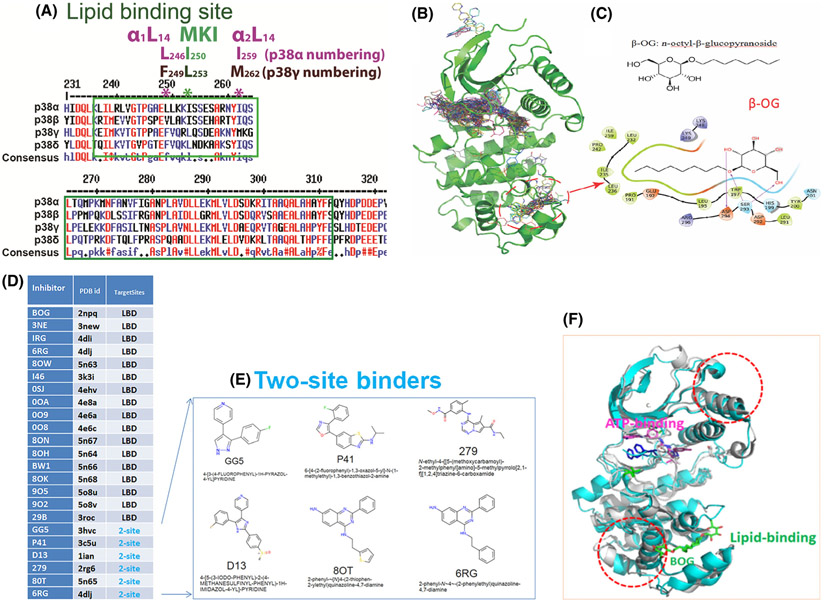
Fig. 1.
Lessons we learnt from p38α on LBD. (A) Consensus amino acids sequences alignment diagram in the lipid-binding domain among four p38 isoforms by clustalW2. MARP insert harbors LBD which is between two αL14 helixes, α1L14 and α2L14. L246 and I259 are two conserved residues of p38α in two L14 loops that indicated in *. Likewise, the counterparts of above two residues in p38 γ are F249 and M262 that are conserved. (B) structures of ligands bound to p38α from 253 PBD entries that have X-ray Crystallography structures. Most of the ligands are single-site binders, either on the ATP-binding site or the lipid-binding site, while some ligands bound to two sites simultaneously. Circled is the area β-OG bound at the lipid-binding pocket. (C) Chemical structure of β-OG (n-octyl-β-glucopyranoside, top) and 2-dimensional (2D) interaction diagram of β-OG in complex with p38α. The hydrophobic tail of β-OG is surrounded by hydrophobic amino acids of p38α (bottom). (D) 17 small ligands of the p38α solely target lipid-binding domain (LBD) and 6 small molecules targets both LBD and ATP binding pocket (data summarized from X-ray crystallography). The column of TargetSites shows the number of sites for a ligand; 2-site (teal color) means that the inhibitor binds at both the lipid-binding site and ATP-binding site simultaneously, and LBD indicates the inhibitor can only bind LBS site of p38α. Only one PDB id is listed for each small molecule. (E) Two site binders of p38α with X-ray crystallography structure consist of 6 molecules: GG5 (PBD: 3hvc); p41 (PBD: 3c5u); D13 (PBD: 1ian); 279 (PBD: 2rg6); 80T (PBD: 5n65); and 6RG (PBD: 4dlj) are ligands for two sites, that is, the ATP binding and the lipid-binding of p38α. (F) β-OG lipid binding of p38α may cause allosteric effects on ATP binding. We superimposed two public PBD datasets from RSCB, the DFG-out conformation of p38α alone (PDB 2baj) is displayed as cyan cartoons; and the inactive p38α bound β-OG as grey cartoons (PDB 3gcv). When β-OG bound, the structure is changed significantly near both the lipid-binding and ATP-binding sites (depicted as dotted red circles), which suggests that the binding of β-OG at the lipid-binding site causes conformational changes near the ATP-binding site and thereby affect its kinase activity allosterically.