FIGURE 3.
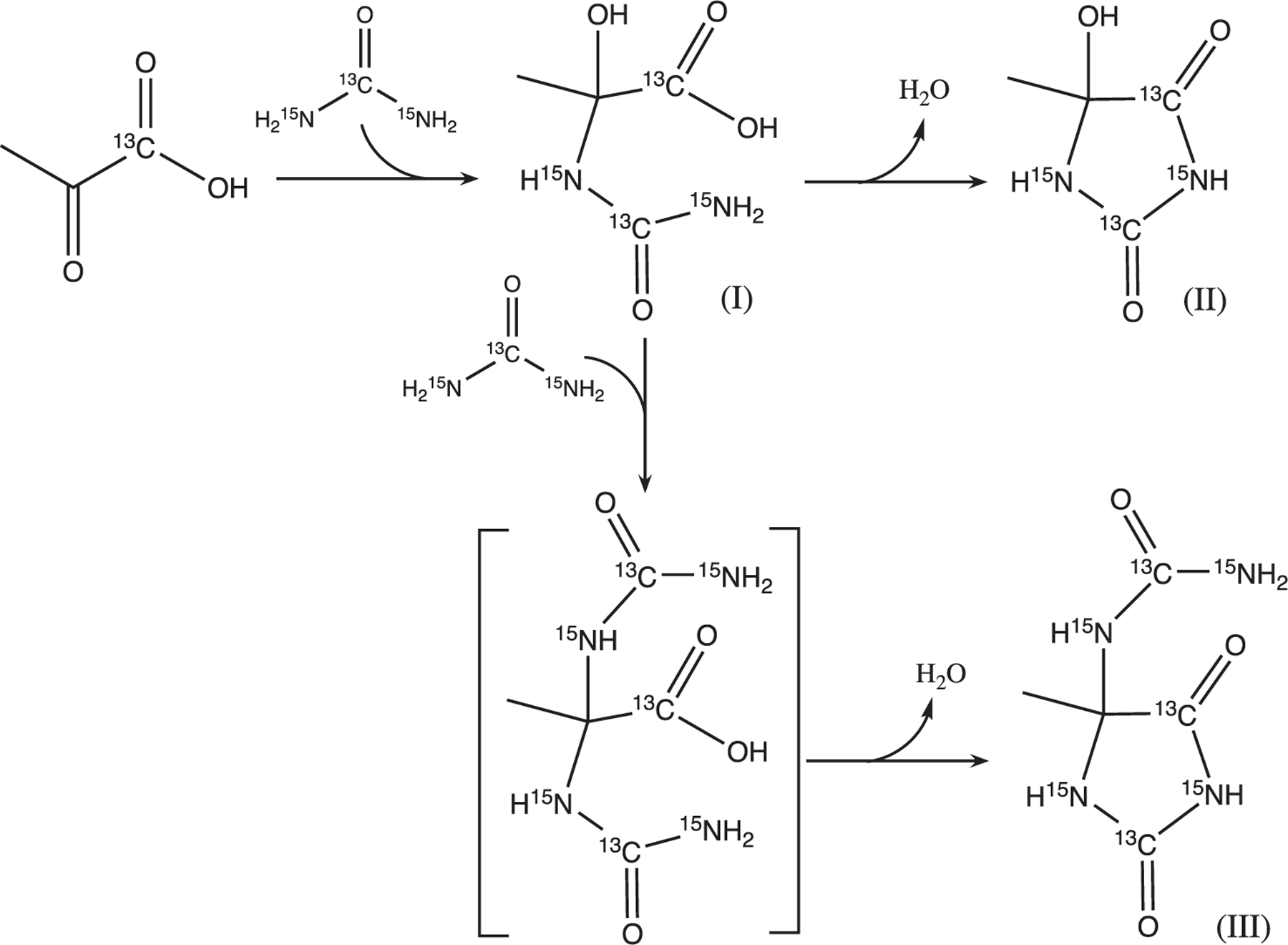
Impurity formation scheme in co-polarized [1-13C]pyruvate and [13C, 15N2]urea. These reactions most likely occur in the cryovial at high-concentration conditions: the nucleophilic addition of urea (nitrogen) to pyruvic acid (C2 carbonyl carbon) yields Impurity I, an intermediate, which further undergoes intramolecular condensation between the carboxylate group and amide group, yielding Impurity II, a hydantoin. Impurity I can also undergo nucleophilic substitution with urea at C2 carbon before intramolecular condensation, yielding Impurity III, another hydantoin
