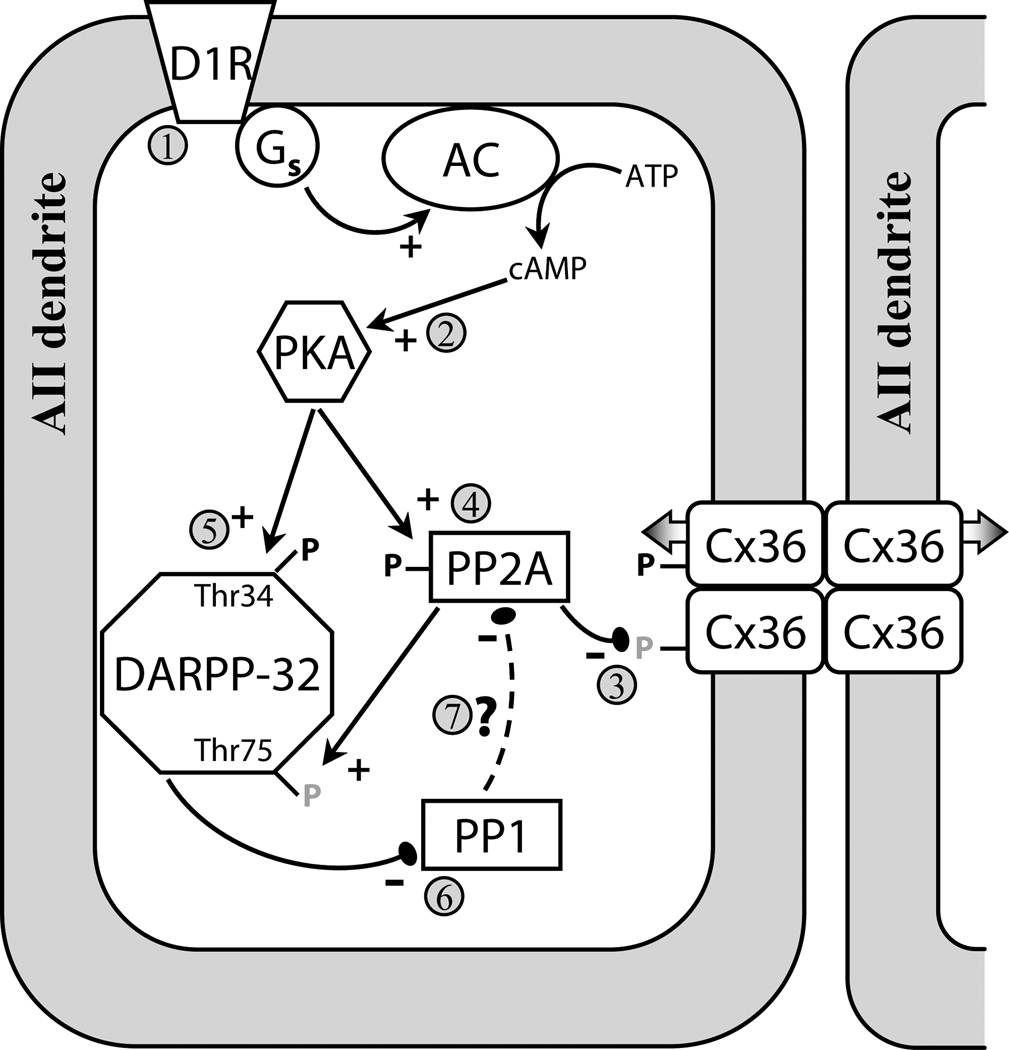
Figure 7. Model of D1R-dependent regulation of Cx36-mediated coupling between AII amacrine cells.
Activation of D1Rs (1) initiates a cascade leading to activation of PKA (2); both D1R and PKA activation are sufficient to uncouple AII amacrine cells (Hampson et al., 1992; Mills and Massey, 1995). In this study we showed that D1R-dependent dephosphorylation (grey P’s) of Ser293 on Cx36 uncouples AII amacrine cells (3). We show that PP2A is required for both D1R- and PKA-stimulated dephosphorylation of Ser293 (4). This provides evidence that the D1R → PKA → PP2A pathway, which was recently described in spiny neurons in the striatum and led to dephosphorylation of Thr75 on DARPP-32 (Ahn et al., 2007), is also present in the AII amacrine cell. AII amacrine cells also express DARPP-32 (Partida et al., 2004; Witkovsky et al., 2007), and dephosphorylation of Thr75 on DARPP-32 facilitates PKA-mediated phosphorylation (black P’s) of Thr34 (5), which converts DAPRR-32 into an inhibitor of PP1 (6) (Svenningsson et al., 2004). We found that PP1 negatively regulates the dephosphorylation of Cx36, possibly by opposing PKA-mediated activation of PP2A (7). Our results indicate that the D1R → PKA → PP2A pathway is not limited to striatal neurons, and may represent a common pathway in neurons expressing D1Rs and DARPP-32.