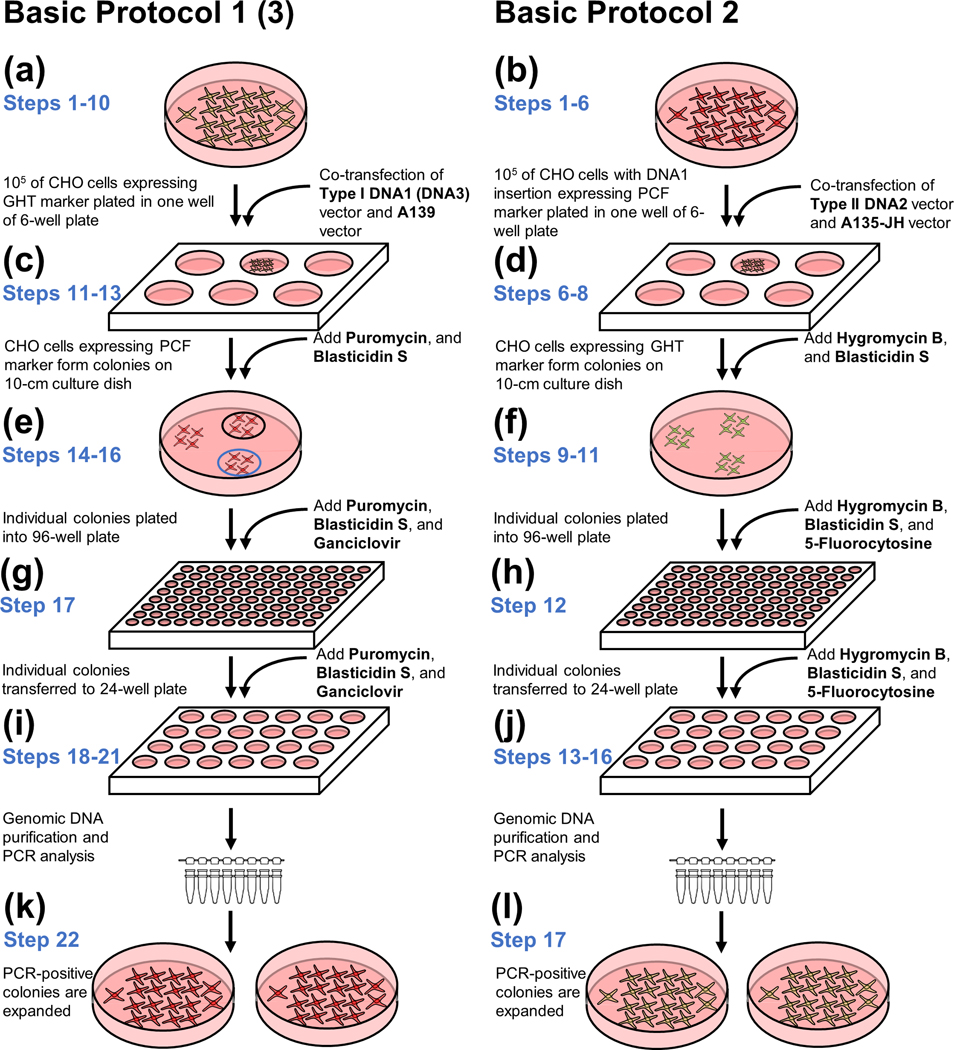
Figure 6.
Cell culture steps in the Basic Protocols. Each round of integration requires similar cell culture procedures. Herein, the steps of the protocols are presented side by side to highlight the key differences between them. The 1st round starts with hamster CHO cells carrying the IIS-alphoidtetO-HAC. Afterwards, any subsequent round starts with the cells obtained during the preceding round. (a-b) The first procedure in each round is co-transfection of a specific carrier vector carrying a genomic DNA fragment, i.e., Type I DNA1, Type II DNA2, and Type I DNA3, along with either (a) A139 or (b) A135-JH vector. (c-d) Cells in with correct integration has occurred exhibit a change in the expression of marker genes (eGFP to mCherry or mCherry to eGFP) and can form colonies under selection with either (c) Puromycin and Blasticidin S or (d) Hygromycin B and Blasticidin S. (e-f) Individual colonies with proper fluorescence are transferred into a 96-well plate. Additional counterselection agents, i.e, (e) Ganciclovir or (f) 5-Fluorocytosine, are supplemented to remove cells with incorrect integration. (g-h) The colonies are transferred to a 24-well plate and grown under selection. (i-j) Genomic DNA is purified from individual colonies and PCR-analyzed to confirm proper integration. (k-l) Colonies with PCR-confirmed integration of the DNA fragment are transferred to 10-cm dishes for FISH analysis and for preparation of frozen stocks.