Abstract
Background:
Neurophysiologic complexity has been shown to decrease during states characterized by a depressed level of consciousness, such as sleep or anesthesia. Conversely, neurophysiologic complexity is increased during exposure to serotonergic psychedelics or subanesthetic doses of dissociative anesthetics. However, the neurochemical substrates underlying changes in neurophysiologic complexity are poorly characterized. Cortical acetylcholine appears to relate to cortical activation and changes in states of consciousness, but the relationship between cortical acetylcholine and complexity has not been formally studied. We addressed this gap by analyzing simultaneous changes in cortical acetylcholine (prefrontal and parietal) and neurophysiologic complexity before, during and after subanesthetic ketamine (10 mg/kg/h) or 50% nitrous oxide.
Methods:
Under isoflurane anesthesia, adult Sprague Dawley rats (n=24, 12 male and 12 female) were implanted with stainless-steel electrodes across the cortex to record monopolar electroencephalogram (0.5–175 Hz, 30 channels), and guide canulae in prefrontal and parietal cortices for local microdialysis quantification of acetylcholine levels. One subgroup of these rats was instrumented with a chronic catheter in jugular vein for ketamine infusion (n=12, 6 male, 6 female). The electroencephalographic data were analyzed to determine subanesthetic ketamine or nitrous oxide-induced changes in Lempel-Ziv complexity and directed frontoparietal connectivity. Changes in complexity and connectivity were analyzed for correlation with concurrent changes in prefrontal and parietal acetylcholine.
Results:
Subanesthetic ketamine produced sustained increases in normalized Lempel-Ziv complexity (0.5–175 Hz, P < .001) and high gamma frontoparietal connectivity (125–175 Hz, P < .001). This was accompanied by progressive increases in prefrontal (104%, P < .001) and parietal (159%, P < .001) acetylcholine levels that peaked after 50 min of infusion. Nitrous oxide induction produced a transient increase in complexity (P < .05) and high gamma connectivity (P < .001), which was accompanied by increases (P < .001) in prefrontal (56%) and parietal (43%) acetylcholine levels. In contrast, the final 50 min of nitrous oxide administration were characterized by a decrease in prefrontal (38%, P < .001) and parietal (45%, P < .001) acetylcholine levels, reduced complexity (P < .001), and comparatively weaker frontoparietal high gamma connectivity (P < .001). Cortical acetylcholine and complexity were correlated in both subanesthetic ketamine (prefrontal: CW r(144)=0.42, P < .001; parietal: CW r(144)=0.42, P < .001) and nitrous oxide (prefrontal: CW r(156)=0.46, P < .001; parietal: CW r(156)=0.56, P < .001) cohorts.
Conclusions:
These data bridge changes in cortical acetylcholine with concurrent changes in neurophysiologic complexity, frontoparietal connectivity, and the level of consciousness.
Introduction
Although the precise neural correlates of consciousness are still a matter of active debate,1,2 there is evidence that cortical neurophysiologic complexity relates to the level of consciousness.3,4 Neurophysiologic complexity has been shown to be depressed during unconscious states such as slow-wave sleep, coma, and anesthesia.5–10 Conversely, psychoactive drugs such as classical serotonergic psychedelics or dissociative NMDA-antagonists are known to enhance complexity, with several studies establishing an association between changes in complexity, cortical connectivity, and reports of alterations to conscious contents.9,11–13 Although cortical complexity has been characterized across a broad spectrum of brain states, there is limited understanding of the relationship between measures of complexity and underlying neurochemical processes within the cortex.
The cortex receives topographically specific cholinergic projections from the basal forebrain.14 Cortical acetylcholine levels are high during states associated with high complexity and the presence of phenomenological content, such as wakefulness, rapid eye movement sleep, or after the administration of serotonergic psychedelics or subanesthetic levels of glutamatergic dissociatives.15–19 Conversely, cortical acetylcholine is suppressed during states of low complexity and reduced conscious content, such as slow-wave sleep or anesthesia.15,20,21
Despite a biologically plausible relationship that might be inferred from past investigations, there has been no study that has characterized – through concurrent measurements – the relationship between neurophysiologic complexity and cortical acetylcholine. Studies characterizing neurophysiologic complexity have often relied on temporospatial analysis of high-density electroencephalographic recordings in human subjects but have not measured concurrent neurochemical changes. On the other hand, studies using animal models typically allow neurochemical analysis but have employed sparse electroencephalographic recordings that prevent the application of temporospatial analyses used in human studies. To address this gap, we developed a novel approach to record high-density (30-channel) intracranial electroencephalogram (EEG) in rats while simultaneously measuring acetylcholine levels in prefrontal and parietal cortices. We then leveraged the NMDA-antagonists ketamine and nitrous oxide as pharmacological tools, due to their unique dose-dependent anesthetic and psychedelic properties. Changes in acetylcholine concentration in prefrontal and parietal cortices were compared with Lempel-Ziv complexity22 before, during, and after administration of subanesthetic ketamine or 50% nitrous oxide. To further characterize cortical dynamics, we computed normalized symbolic transfer entropy, an information theoretic measure to estimate directed connectivity,21,23,24 within gamma bandwidths between frontal and parietal cortices. We report that changes in cortical acetylcholine levels during subanesthetic ketamine or 50% nitrous oxide exposure correlate with changes in neurophysiologic complexity and high gamma connectivity.
Methods
Rats
The study was approved by the Institutional Animal Care and Use Committee (University of Michigan, Ann Arbor, Michigan, USA) and was performed in accordance with the Guide for the Care and Use of Laboratory Animals (8th Edition, The National Academies Press, Washington D.C.), as well as ARRIVE guidelines. Adult male and female Sprague-Dawley rats (n = 24, 12 male:12 female, 300–350 g, Charles River Laboratories, MA) were used. The rats were housed in a temperature- and light-controlled facility (12 h light: 12 h dark cycle, lights on at 8:00 am) with ad libitum access to food and water.
Surgical Procedures
The surgical procedures have been described in our recent studies21,24,25 and are provided in detail in the supplemental content (Supplemental Digital Content). In brief, under surgical isoflurane anesthesia, rats were implanted with stainless steel screw electrodes to record high-density EEG from across the cortex (Supplemental Digital Content, Figure 1) and microdialysis guide tubes aimed at prefrontal cortex (prelimbic region) and parietal cortex (somatosensory barrel field region). In a subset of rats (n=12, 6 male, 6 female), an indwelling catheter was surgically positioned into the jugular vein to allow for intravenous infusion of ketamine.
Experimental design
Schematics depicting the design for ketamine and nitrous oxide experiments are illustrated in Figure 1A and 1B, respectively. The experiments were conducted after at least 10 days of post-surgical recovery. Equal sized cohorts of rats (n=12, 6 male, 6 female) were used for ketamine and nitrous oxide experiments. EEG data were recorded continuously throughout the experiment while microdialysis samples were collected in 12.5 min epochs. The EEG was recorded, and dialysis samples were collected for 50 min (4 microdialysis epochs) during freely moving baseline wake condition. The EEG was monitored in real-time by the experimenter and gentle tapping on the recording chamber was used to maintain a constant state of wakefulness. Following baseline recording, rats were either connected to an intravenous catheter line to allow for sustained infusion of ketamine at 10 mg/kg/h or were sealed in an air-tight chamber to allow for delivery of a mixture of 50% nitrous oxide and 50% oxygen into the recording chamber (12 L/min). The EEG and dialysis samples were then collected for a period of 62.5 min (5 microdialysis epochs) during administration of subanesthetic ketamine or nitrous oxide. Ketamine infusion or nitrous oxide exposure was then stopped, and data were collected for another 62.5 min (5 microdialysis epochs) during post-drug recovery period. At the conclusion of data collection, the sites of microdialysis were histologically verified (Supplemental Digital Content, Figure 2).
Figure 1.
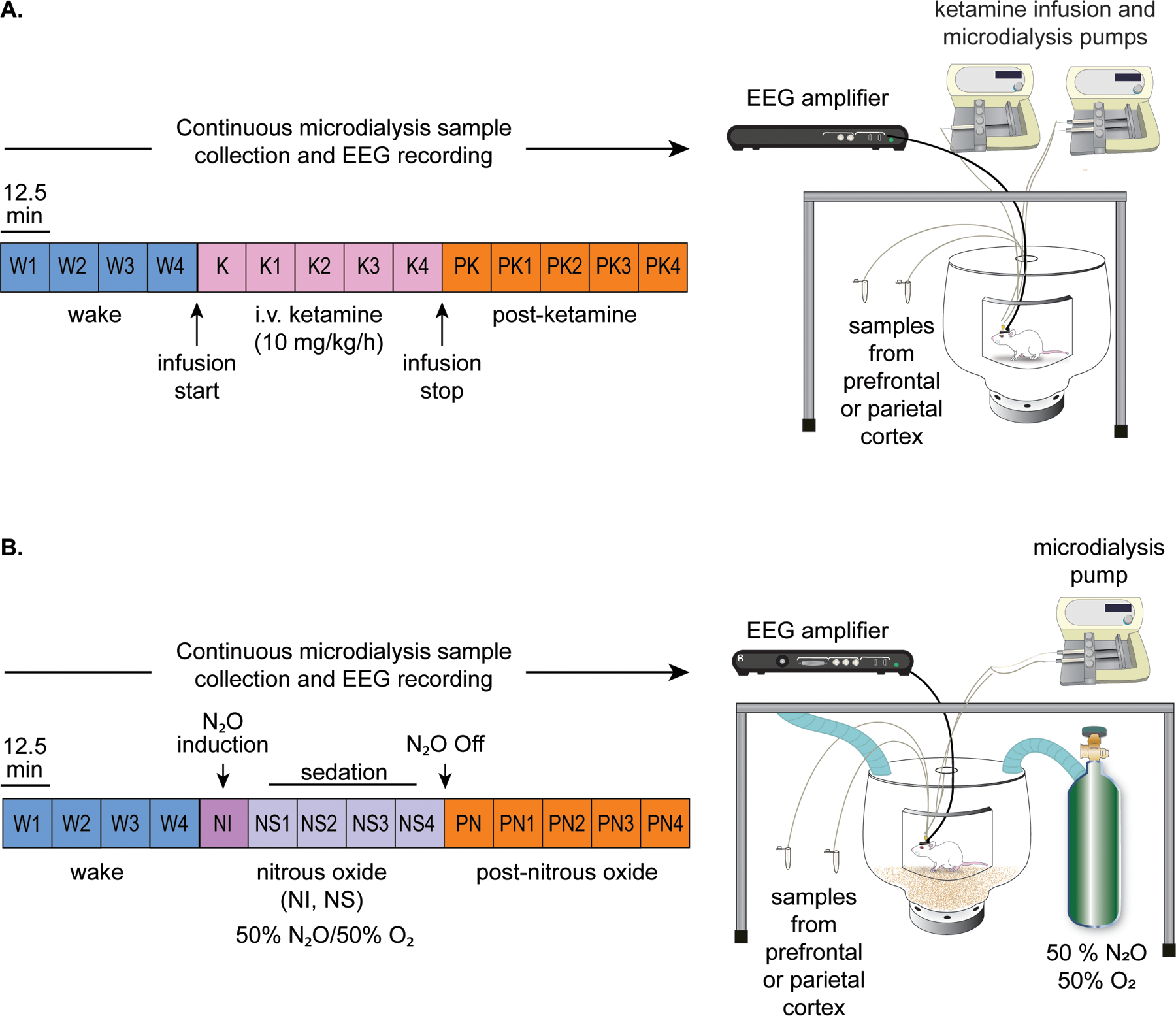
Schematics illustrating the experimental set-up and timeline for ketamine (A) and nitrous oxide (B) experiments. The EEG data and microdialysis samples from prefrontal and parietal cortices were collected simultaneously and continuously but the microdialysis samples were collected in 12.5 min bins. Each colored box represents one microdialysis epoch. Samples were collected during freely moving baseline wake (W) condition, continuous subanesthetic ketamine infusion at 10 mg/kg/h (K), or post-ketamine recovery period (PK). The data collection was performed similarly for the nitrous oxide (N2O) cohort, with epochs corresponding to the wake state (W), 50% nitrous oxide induction (NI), 50% nitrous oxide sedation (NS), and post-nitrous oxide recovery period (PN).
EEG data acquisition and quantification of cortical acetylcholine levels
Monopolar EEG signals, referenced to an electrode over the nasal sinus, were acquired (0.1–500 Hz, 1 kHz sampling rate) from across the cortex. Prefrontal and parietal microdialysis samples were analyzed with high-performance liquid chromatography and electrochemical detection for quantification of acetylcholine levels. The detailed methodology is provided in the Supplemental Digital Content.
Lempel-Ziv complexity analysis
Lempel-Ziv complexity (LZs) was used to approximate temporospatial EEG complexity. LZs is a method of symbolic-sequence analysis that assesses the algorithmic complexity (i.e. diversity, compressibility) of finite sequences such as EEG time series.22 LZs analysis was conducted as outlined in previous studies from our laboratory9,10,24 and others.7,8,12,13 To control for any potential bias in the resultant LZs values due to the frequency content of the EEG signal, we normalized LZs by the average of n=50 surrogate datasets in which the spectral profile were identical to the original signal, but the phase-information was maximally randomized.7–10,12,24 This excludes the possibility that changes in complexity reflected in the normalized metric of LZs (denoted LZsN) are attributable only to the spectral contents of the signal.
Frontoparietal directed connectivity: normalized symbolic transfer entropy analysis
We used normalized symbolic transfer entropy (NSTE) to assess the directed connectivity between frontal and parietal cortices. The analysis methods have been described in our previous studies21,23,24 and are provided in the Supplemental Digital Content. We focused on changes in directed connectivity between ipsilateral frontal and parietal channels and in three gamma bandwidths [low gamma (25–55 Hz), mid gamma (85–125 Hz), and high gamma (125–175 Hz)], because previous work from our laboratory has demonstrated directed frontoparietal connectivity in high gamma bands to be a correlate of wakefulness.21
Statistical Analyses
All statistical analyses were performed using R software26 in consultation with the Consulting for Statistics, Computing, and Analytics Research Core at the University of Michigan, Ann Arbor.
Our primary outcome measures were changes in cortical acetylcholine, Lempel-Ziv complexity, and frontoparietal connectivity. No a priori power analysis for sample size selection was performed. The sample size was informed by our previously published similar studies19,21,24,25 in which cortical acetylcholine, directed connectivity, or complexity were analyzed using identical analytic methodologies, and in which we found that sample sizes of ≤ 11 were sufficient in detecting significant differences in these outcome measures across experimental conditions. The first ketamine infusion epoch was excluded from EEG and neurochemical analyses due to the time required for the drug to traverse dead space in the catheter and reach blood circulation. For the rest of the epochs in ketamine experiments, a within-subjects design was utilized in which each rat contributed 4 wake datapoints, 4 ketamine infusion datapoints, and 4 post-ketamine datapoints for each measure. As opposed to intravenous administration of ketamine, which required 5–7 minutes to traverse the catheter, nitrous oxide exposure was quick, and the recording chamber was filled within 1 min of the start of nitrous oxide delivery. However, the rats showed an acute response to nitrous oxide, which was primarily limited to the first 12.5 min of nitrous oxide delivery and differed from the subsequent 50 mins of exposure. Therefore, we analyzed the first epoch (12.5 min) of nitrous oxide exposure separately as “nitrous induction” while the rest of the 4 epochs (50 min) were categorized as nitrous “sedation” and analyzed as a separate block. For nitrous oxide experiments, we implemented a within-subjects design in which each rat contributed 4 wake datapoints, 1 nitrous oxide induction datapoint, 4 nitrous oxide sedation datapoints, and 4 post-nitrous oxide datapoints for each EEG measure and acetylcholine analysis. The statistical analyses for ketamine experiments were run on 5 min segments of noise-free EEG from each 12.5 min epoch, while for nitrous oxide experiments, 2 min segments of noise-free EEG were selected. The choice to use shorter EEG epochs for nitrous oxide experiments was due to the prevalence of noise in the EEG signal driven by sporadic chewing or “bruxing” behavior. To exclude mixed states, the first post-ketamine and first post-nitrous oxide epochs were excluded from our analyses. Thus, each rat in the ketamine experiments contributed 12 EEG and microdialysis datapoints to the statistical analysis, while each rat in the nitrous experiments contributed 13 data points. A linear mixed model was utilized in which “Drug State” was treated as a fixed factor. We also included “Subject Sex” as a fixed factor to account for any influences of sex on drug response. “Subject” was treated as a random intercept in the model to account for inter-subject variability. An alpha threshold of P < .05 was selected and Tukey’s post hoc test was used to correct for multiple pairwise comparisons (paired t-test) between states. Same data analysis plan was followed to quantify the changes in spectral power. The methodology for power spectral analysis and the results are reported in Supplemental Digital Content, Figure 3. To assess correlations between cortical complexity, connectivity, and acetylcholine levels, we employed a Pearson correlation-based method, optimized for analysis of clustered repeated-measures data.27 The data are provided as box and whisker plots with median, interquartile range, minimum and maximum values, and the individual data points for each subject superimposed on the plots. The mean, standard deviation, and F-statistics for statistical comparisons are provided in Supplemental Digital Content, Tables 1–6.
Results
Subanesthetic ketamine infusion produced a sustained increase in acetylcholine levels in prefrontal and parietal cortices
Intravenous infusion of subanesthetic ketamine resulted in a consistent increase in acetylcholine levels relative to wake state in both prefrontal [104%, t(130)=12.49, P < .001, Fig. 2A] and parietal [159%, t(130)=13.72, P < .001, Fig. 2B] cortices, lasting all 4 epochs and reaching maximum levels in the final 12.5 minutes of infusion. During subanesthetic ketamine infusion, rats displayed stereotypic head bobbing and circling behavior, interrupted by periodic bouts of ataxia. As compared to the wake state, acetylcholine levels in both prefrontal and parietal cortices remained elevated during post-ketamine recovery period [21%, prefrontal: t(130)=3.54, P < .01, Fig. 2A; parietal: 23%, t(130)=2.546, P = .03, Fig. 2B], with cortical acetylcholine progressively declining 12.5 min after the cessation of the ketamine infusion. The acetylcholine levels during the post-ketamine recovery period were significantly lower than that during subanesthetic ketamine infusion [prefrontal: −26%, t(130)=−8.95, P < .001, Fig. 2A; −41%, parietal: t(130)=−11.17, P < .001, Fig. 2B].
Figure 2.
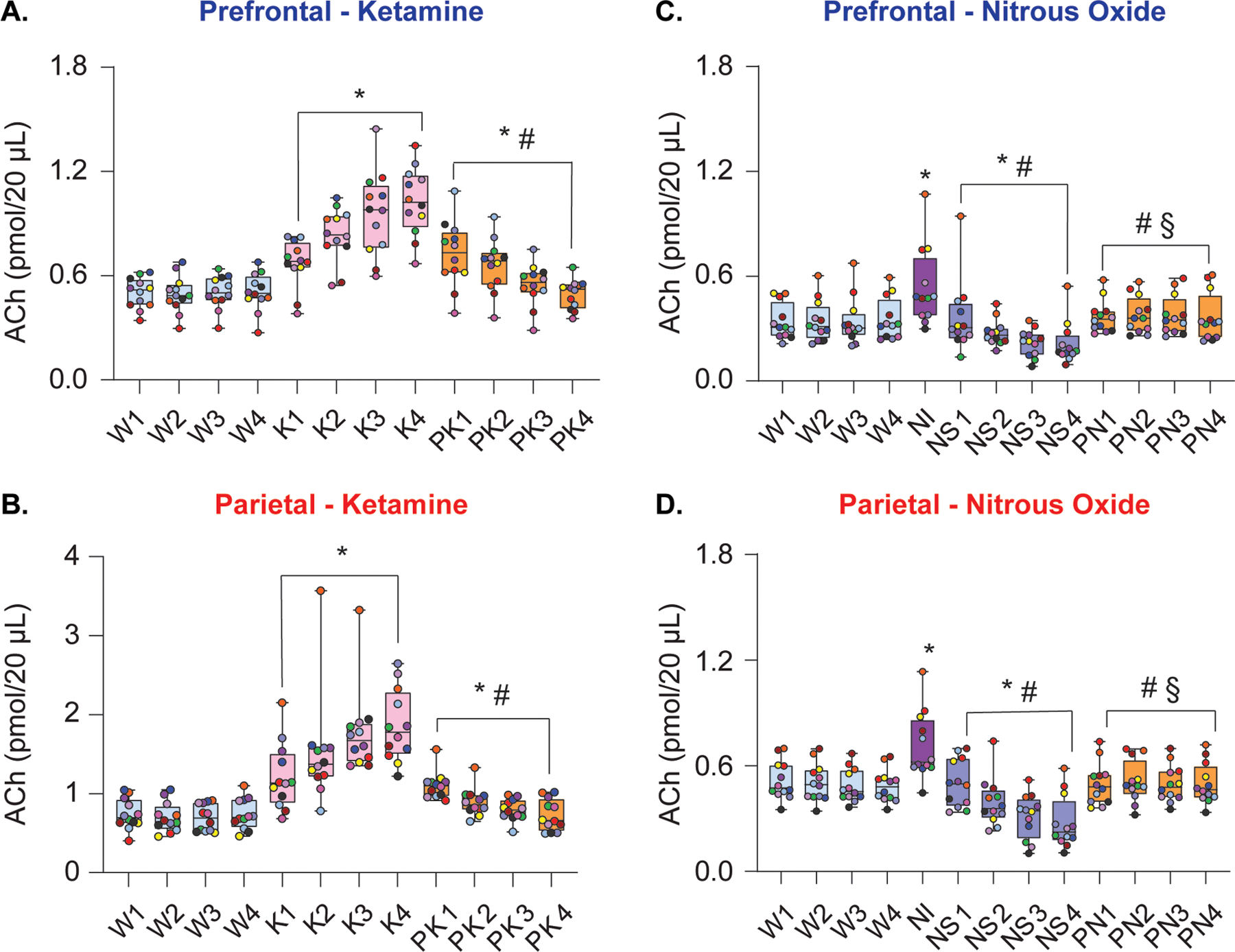
Subanesthetic ketamine and nitrous oxide administration produced differential effects on cortical acetylcholine levels. Subanesthetic ketamine infusion produced sustained increase in prefrontal (A) and parietal (B) acetylcholine levels. During post-ketamine recovery, cortical acetylcholine levels remained elevated compared to wakefulness, though this effect was largely driven by the first 2 post-ketamine epochs (A, B). In contrast, 50% nitrous oxide treatment transiently increased prefrontal and parietal acetylcholine levels during the first 12.5 min of exposure (nitrous induction), followed by a progressive decline in acetylcholine during nitrous oxide sedation phase (C, D). Cortical acetylcholine levels during post-nitrous oxide recovery did not significantly differ from those observed during baseline wake state (C, D). A linear mixed model with a random intercept for each rat was used for statistical comparisons. Post hoc pairwise tests between states were performed with single-step correction for multiple comparisons via Tukey’s Test. The box plots show the median (horizontal bar) and interquartile range for averaged data over all 12 subjects at each epoch. The whiskers represent the minimum and maximum values within each epoch. The data for each subject are displayed by colored dots, with each color corresponding to a single subject across all epochs. ACh – acetylcholine, K – subanesthetic ketamine infusion, NI – nitrous oxide induction, NS – nitrous oxide sedation, PK – post-ketamine recovery, PN – post-nitrous oxide, W – wake. *Significant compared to wake, #significant compared to subanesthetic ketamine infusion or nitrous oxide induction, §significant compared to nitrous oxide sedation. The statistical comparisons are shown at P < .05. The exact P values are provided in the text in the results section. The mean, SD, and F-statistics for statistical comparisons are provided in Supplemental Digital Content, Tables 1–2.
Nitrous oxide exposure produced state-dependent effects on acetylcholine levels in prefrontal and parietal cortices
Nitrous oxide exposure resulted in state-dependent effects on acetylcholine in prefrontal and parietal cortices that depended on the depth of sedation, which increased with the duration of exposure. During the first 12.5 min of nitrous oxide exposure, hereafter referred to as nitrous induction (NI), rats showed active behavior such as grooming, stereotypic head bobbing, and uncoordinated locomotion. Acetylcholine levels in both prefrontal and parietal cortices were significantly elevated relative to wake [prefrontal: 56%, t(141)=7.97, P < .001, Fig. 2C; parietal: 43% t(141)=7.39, P < .001, Fig. 2D]. The following 50 min of nitrous oxide exposure, hereafter referred to as nitrous sedation (NS), were marked by periodically quiescent behavior interrupted by active and purposeful behaviors such as slow locomotion, grooming, or pica-like consumption of bedding. The requirement to have a sealed recording chamber precluded any behavioral manipulation to assess the level of sedation. However, despite relatively quiescent behavior, rats clearly retained righting reflex throughout nitrous oxide treatment, displayed sporadic spontaneous behavior, and were easily aroused by tapping on the wall of recording chamber. This was accompanied by a progressive decline in prefrontal [38%, t(141)=−5.01, P < .001, Fig. 2C] and parietal [45%, t(141)=−7.07, P < .001, Fig. 2D] acetylcholine, relative to wake state. Cortical acetylcholine showed a rapid return to pre-nitrous levels during post-nitrous recovery and did not statistically differ from the wake state [prefrontal: t(141)=1.86, P = .2, Fig 2C; parietal: t(141)=0.01, P = 1, Fig. 2D].
Subanesthetic ketamine infusion induced persistent increase in temporospatial EEG complexity
To assess the effect of subanesthetic ketamine infusion on temporospatial EEG complexity independent of the power spectrum, normalized Lempel-Ziv complexity (LZsN) was computed for EEG epochs before, during, and after ketamine infusion. As compared to baseline wake state, LZsN was significantly higher during subanesthetic ketamine [t(130)=9.77, P < .001, Fig. 3A]. During post-ketamine recovery period, LZsN values decreased relative to ketamine infusion [t(130)=−8.89, P < .001, Fig. 3A], returning to values that did not significantly differ from the wake state [t(130)=0.88, P = .7, Fig. 3A]. A topographic plot illustrating the degree of change in LZsN during subanesthetic ketamine relative to wake in each EEG channel (as calculated by the t-statistic) is depicted in figure 3B, with regions colored red representing the areas with the greatest magnitude of change in LZsN. EEG channels located within the frontal, posterior parietal, and occipital cortex showed the most statistically significant changes in LZsN during subanesthetic ketamine infusion, as compared to the wake state (Fig. 3B).
Figure 3.
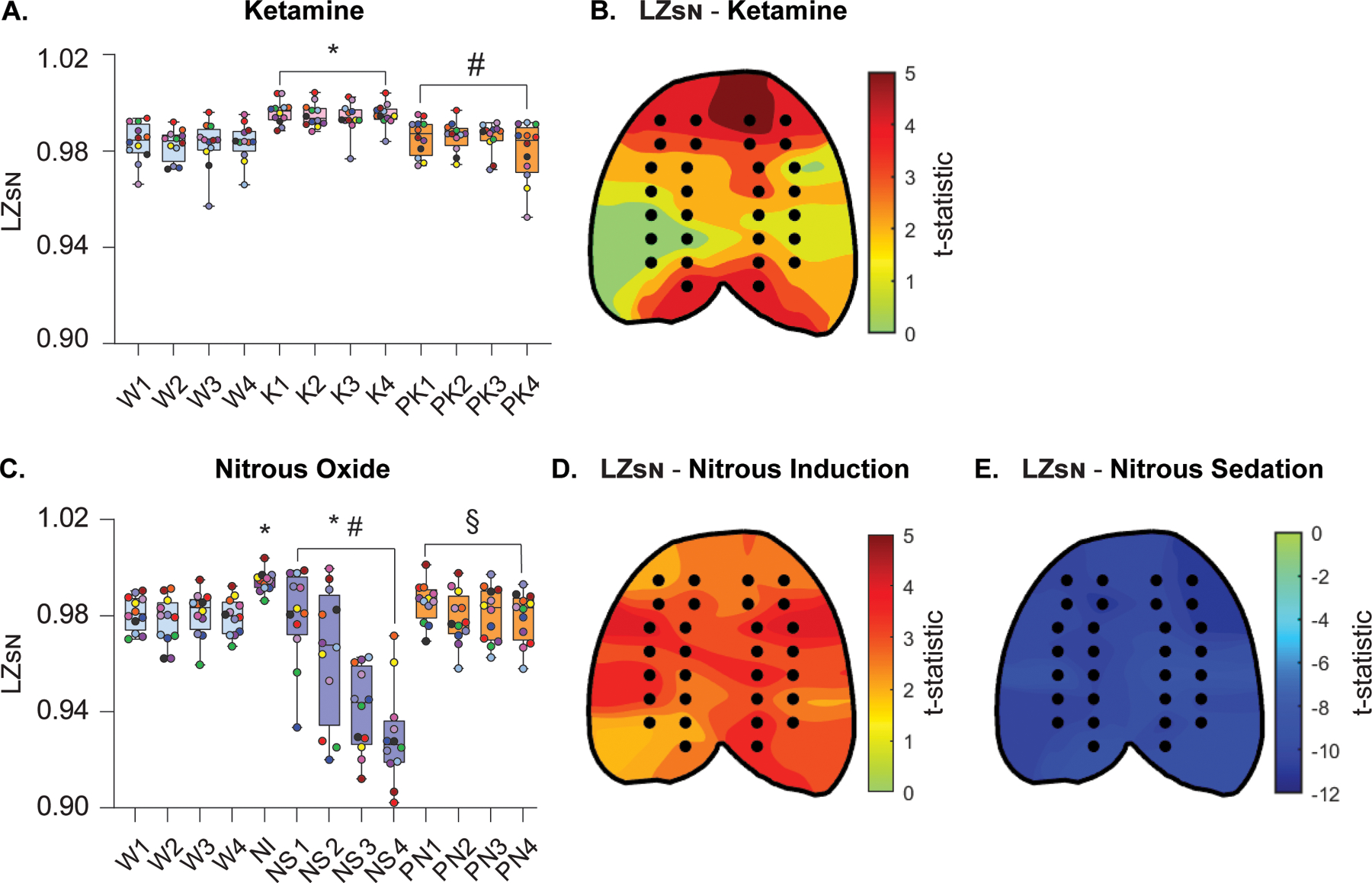
Changes in temporospatial EEG complexity during subanesthetic ketamine infusion and nitrous oxide exposure mirror concomitant changes in cortical acetylcholine levels. Normalized Lempel-Ziv complexity (LZsN) was significantly increased during subanesthetic ketamine infusion, returning to wake levels after the infusion was stopped (A). Changes in LZsN during subanesthetic ketamine infusion were also quantified at the level of single channels (black dots in the topographic plots), demonstrating that the most pronounced changes in temporal complexity, relative to wake, occurred in frontal, posterior parietal, and occipital channel clusters (B). LZsN significantly increased during the first 12.5 min of nitrous oxide exposure (induction phase) as compared to baseline wake state (C). During the subsequent nitrous oxide sedation phase, the LZsN declined to levels significantly lower than that observed during baseline wake. LZsN returned to levels comparable to baseline wakefulness during post-nitrous oxide recovery (C). The most significant increases in temporal complexity during nitrous oxide induction, as compared to baseline wake state, were located in frontotemporal, parietal, and occipital regions (D). Nitrous oxide sedation was characterized by a significant decrease in temporal complexity across the entire cortical surface (E). A linear mixed model with a random intercept for each rat was used for statistical comparisons. Post hoc pairwise tests between states were performed with single-step correction for multiple comparisons via Tukey’s Test. The box plots show the median (horizontal bar) and interquartile range for averaged data over all 12 subjects at each epoch. The whiskers represent the minimum and maximum values within each epoch. The data for each subject are displayed by colored dots, with each color corresponding to a single subject across all epochs. K – subanesthetic ketamine infusion, LZSN - normalized Lempel-Ziv complexity, NI – nitrous oxide induction, NS – nitrous oxide sedation, PK – post-ketamine recovery, PN – post-nitrous oxide, W – wake. *Significant compared to wake, #significant compared to subanesthetic ketamine infusion or nitrous oxide induction, §significant compared to nitrous oxide sedation. The statistical comparisons are shown at P < .05. The exact P values are provided in the text in the results section. The mean, standard deviation, and F-statistics for statistical comparisons are provided in Supplemental Digital Content, Tables 3–4.
Nitrous oxide exposure caused state-dependent changes in temporospatial EEG complexity
Mirroring its effects on cortical acetylcholine, nitrous oxide exposure produced changes in EEG complexity that largely depended on the duration of exposure. The LZsN during the first 12.5 min of nitrous oxide exposure (i.e., the induction phase) was found to be significantly higher than that observed during baseline wake state [t(141)=2.64, P = .04, Fig. 3C]. However, in the following 50 min of nitrous oxide exposure, the animals showed behavioral signs of sedation (i.e., reduced movement, slow stereotypic head bobbing, and sporadic grooming) accompanied with progressive decline in LZsN values, which were significantly lower than that observed during both wakefulness [t(141=−7.34, P < .001, Fig. 3C] and the nitrous oxide induction phase [t(141=−7.28, P < .001, Fig. 3C]. During the post-nitrous recovery period, LZsN values returned to levels that were significantly greater than nitrous oxide sedation [t(141)=7.8, P < .001, Fig. 3C], but did not significantly differ from levels observed during baseline wake state [t(141)=0.45, P = 1, Fig. 3C]. Figures 3D and 3E illustrate topographic maps of the t-statistic representing changes in LZsN relative to wake during nitrous oxide treatment within each EEG channel, with red regions representing channels with the most significant increases in LZsN and the darkest blue regions representing the most significant decreases in LZsN. Nitrous oxide induction caused a broad increase in neurophysiologic complexity across the EEG montage, with channels within the temporal, parietal, and occipital regions showing the most significant increases in LZsN, as compared to the wake state (Fig. 3D). In contrast, nitrous oxide sedation in the following 50 min caused global suppression of LZsN relative to the wake state, with significant decreases in EEG complexity spanning the entirety of the cortex (Fig. 3E).
Prefrontal and parietal acetylcholine levels correlate with the changes in cortical complexity
To assess the correlation between changes in prefrontal and parietal acetylcholine levels and temporospatial EEG complexity, the cluster-weighted marginal correlation was computed for both ketamine and nitrous oxide groups. Prefrontal and parietal acetylcholine levels for each microdialysis epoch were compared with the concomitant changes in LZsN measured across all 144 individual datapoints in ketamine experiments, or all 156 datapoints in nitrous oxide experiments, with data dependencies based on individual rats factored into the analysis. Acetylcholine levels in the prefrontal and parietal cortices showed significant positive correlations with LZsN both in the ketamine group [prefrontal: CW r(144)=0.42, P < .001, Fig. 4A; parietal: CW r(144)=0.49, P < .001, Fig. 4B] and the nitrous oxide group [prefrontal: CW r(156)=0.46, P < .001, Fig. 4C; parietal: CW r(156)=0.56, P < .001, Fig. 4D].
Figure 4.
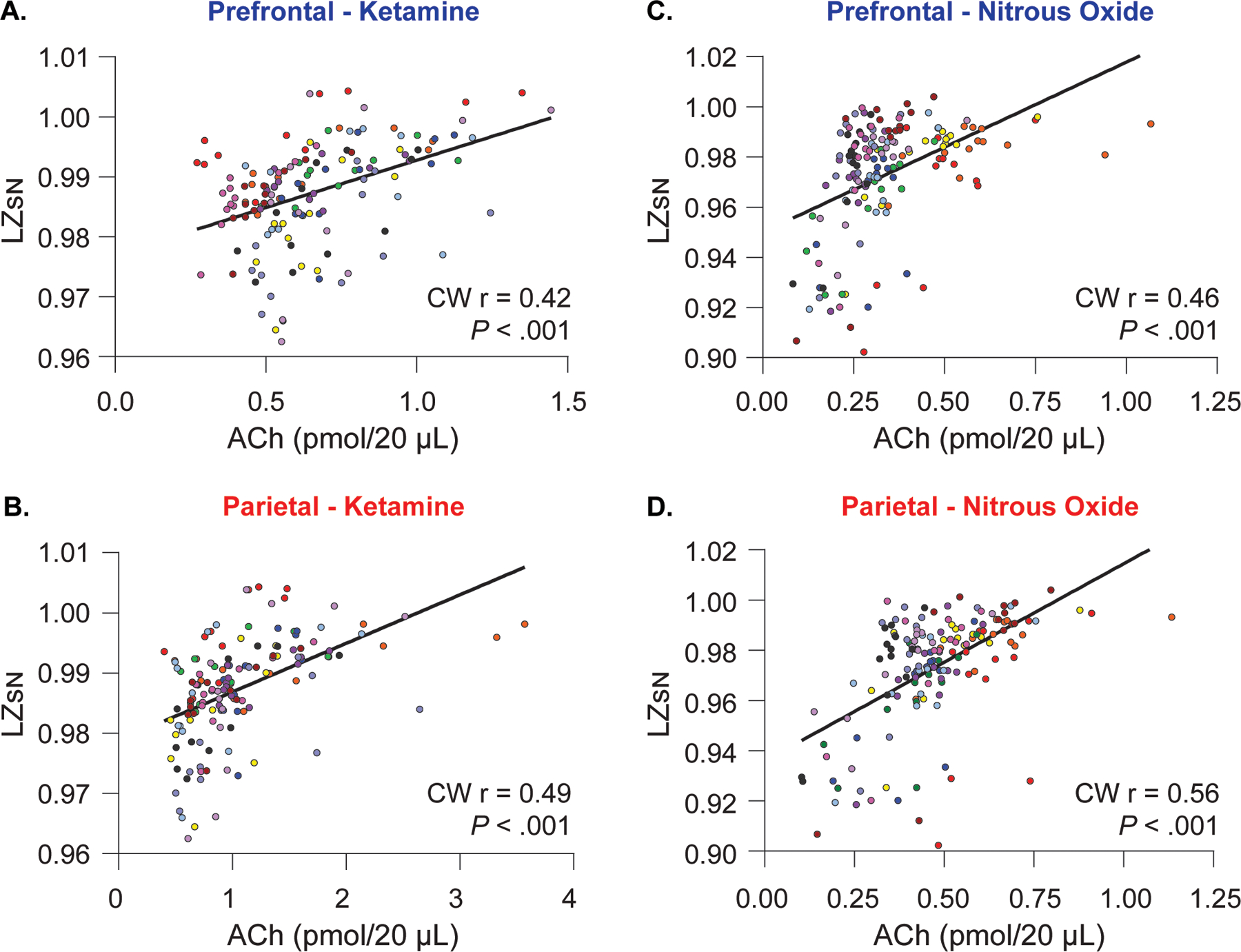
Relationship between cortical acetylcholine and temporospatial EEG complexity. Changes in prefrontal and parietal acetylcholine levels were significantly correlated with changes in temporospatial EEG complexity in the subanesthetic ketamine infusion (A, B) and nitrous oxide exposure (C, D) cohorts. The data for each subject are displayed by colored dots, with each color corresponding to a single subject across all epochs. To account for clustering of the data within each rat, we calculated the cluster-weighted marginal correlation. ACh – acetylcholine, LZSN – normalized Lempel-Ziv complexity, CW r – cluster-weighted marginal correlation. The line represents points of best fit.
Effects of subanesthetic ketamine on frontoparietal connectivity in gamma bandwidths
Normalized symbolic transfer entropy (NSTE) was computed across low (25–55 Hz), mid (85–125 Hz), and high gamma (125–175 Hz) bandwidths between ipsilateral frontal and parietal channels as an estimate of directed feedback (frontal to parietal) and feedforward (parietal to frontal) connectivity. Subanesthetic ketamine suppressed low and mid gamma frontoparietal connectivity in both feedback [low: t(130)=−6.89, P < .001, Fig. 5A; mid: t(130)=−15.12, P < .001, Fig. 5C] and feedforward directions [low: t(130)=−8.20, P < .001, Fig 5B; mid: t(130)=−14.90, P < .001, Fig. 5D]. Conversely, high gamma frontoparietal connectivity was significantly increased during subanesthetic ketamine in both feedback [t(130)=17.59, P < .001, Fig. 5E] and feedforward [t(130)=17.24, P < .001, Fig. 5F] directions. As compared to wake state, post-ketamine recovery was characterized by reduced frontoparietal connectivity in the mid gamma band [feedback: t(130)=−10.54, P < .001, Fig. 5C; feedforward: t(130)=−12.79, P < .001, Fig. 5D] while the low and high gamma connectivity returned to levels that did not significantly differ from that observed during the baseline wake state. In the low gamma band, feedback and feedforward connectivity were elevated relative to subanesthetic ketamine [feedback: t(130)=3.85, P < .001, Fig. 5A; feedforward: t(130)=6.24, P < .001, Fig 5B]. High gamma connectivity during post-ketamine recovery remained attenuated as compared to subanesthetic ketamine [feedback: t(130)=−18.02, P < .001, Fig. 5E; feedforward: t(130)=−17.89, P < .001, Fig. 5F]. The correlations between frontoparietal connectivity in gamma bandwidths and acetylcholine levels in prefrontal and parietal cortices are reported in Supplemental Digital Content, Table 7.
Figure 5.
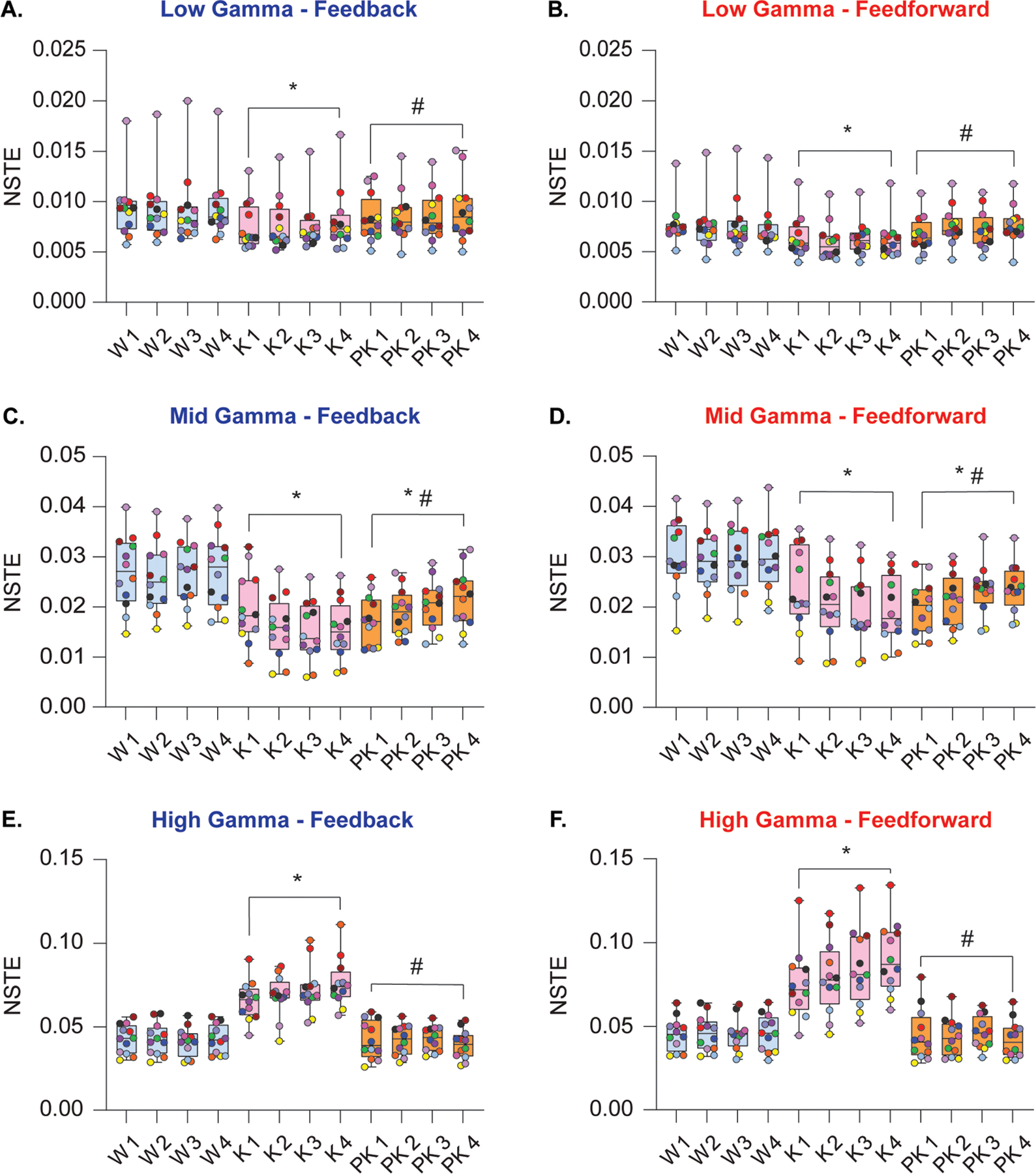
Effect of subanesthetic ketamine infusion on directed frontoparietal connectivity in gamma bandwidths. Subanesthetic ketamine infusion significantly decreased frontoparietal connectivity in the low gamma bandwidth (25–55 Hz) in both feedback and feedforward directions relative to baseline wake state (A, B). Frontoparietal connectivity in the mid gamma bandwidth (85–125 Hz) decreased in feedback and feedforward directions during subanesthetic ketamine and remained depressed relative to baseline wakefulness during post-ketamine recovery (C, D). Feedback and feedforward connectivity between frontal and parietal cortices was significantly increased in the high gamma bandwidth (125–175 Hz) during subanesthetic ketamine treatment, returning to levels comparable to baseline wakefulness during post-ketamine recovery (E, F). A linear mixed model with a random intercept for each rat was used for statistical comparisons. Post hoc pairwise tests between states were performed with single-step correction for multiple comparisons via Tukey’s Test. The box plots show the median (horizontal bar) and interquartile range for averaged data over all 12 subjects at each epoch. The whiskers represent the minimum and maximum values within each epoch. The data for each subject are displayed by colored dots, with each color corresponding to a single subject across all epochs. K – subanesthetic ketamine infusion, PK – post-ketamine recovery, NSTE – normalized symbolic transfer entropy, W – wake. *Significant compared to wake, #significant compared to subanesthetic ketamine infusion. The statistical comparisons are shown at P < .05. The exact P values are provided in the text in the results section. The mean, SD, and F-statistics for statistical comparisons are provided in Supplemental Digital Content, Table 5.
Effects of nitrous oxide exposure on frontoparietal connectivity in gamma bandwidths
Nitrous oxide exposure produced no significant effect on low gamma frontoparietal connectivity in the first 12.5 min induction period [feedback: t(141)=0.44, P = 1, Fig. 6A; feedforward: t(141)=1, P = .7, Fig. 6B]. However, the following 50 min of nitrous oxide sedation significantly increased low gamma frontoparietal connectivity in both feedforward and feedback directions, as compared to the wake state [feedback: t(141)=6.41, P < .001, Fig. 6A; feedforward: t(141)=6.84, P < .001, Fig. 6B]. As compared to nitrous oxide sedation, the post-nitrous recovery was characterized by significant attenuation of both feedback and feedforward low gamma frontoparietal connectivity [feedback: t(141)=−6.18, P < .001, Fig. 6A; feedforward: t(141)=3.33, P = .005, Fig. 6B], with no significant difference as compared to the baseline wake levels [low gamma feedback: t(141)=0.24, P = 1, Fig. 6A; low gamma feedforward: t(141)=0.5, P = .9, Fig. 6B].
Figure 6.
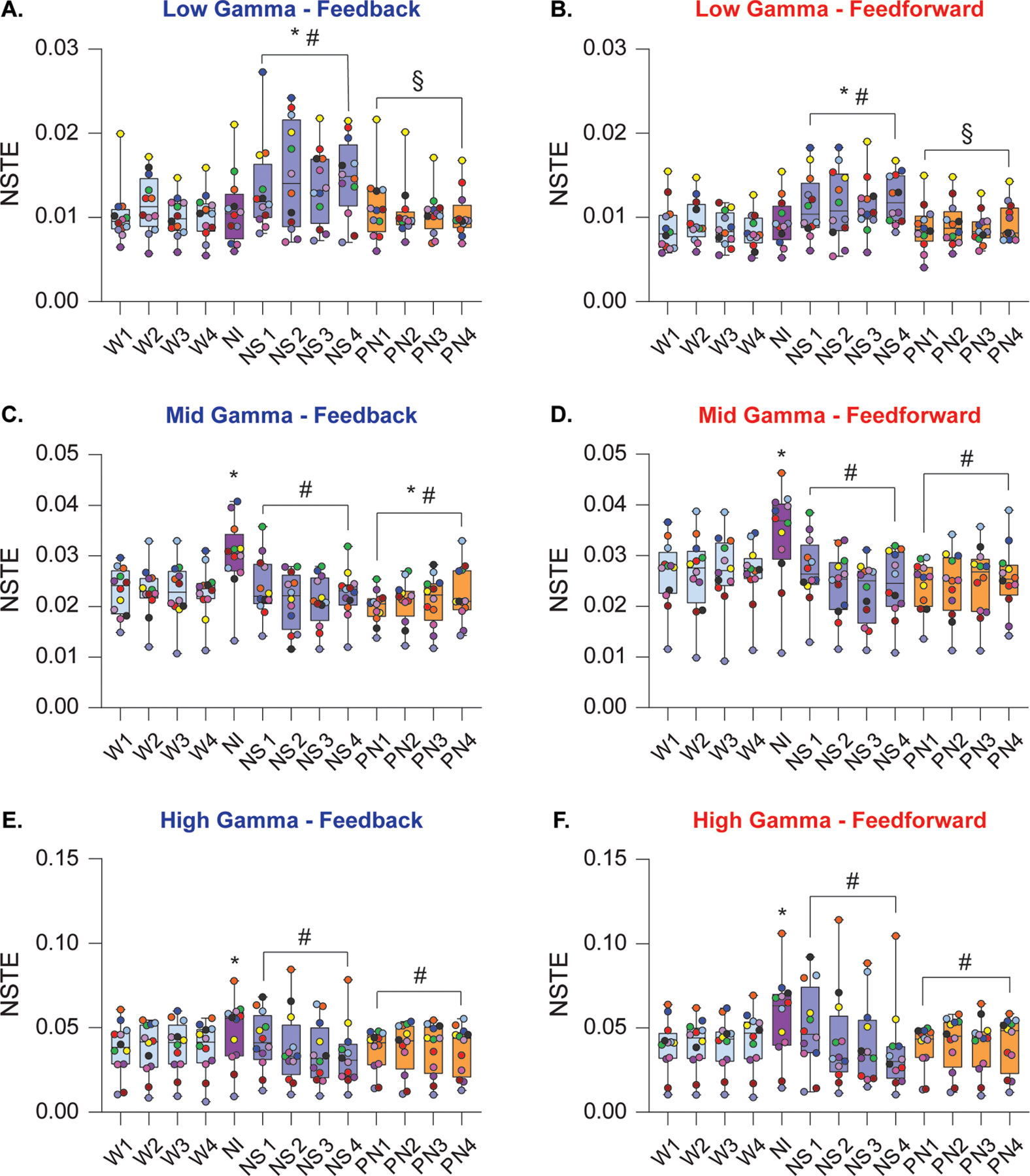
Effects of nitrous oxide exposure on directed frontoparietal connectivity in gamma bandwidths. During nitrous oxide induction, frontoparietal connectivity in the low gamma bandwidth (25–55 Hz) did not differ from wake (A, B). However, feedback and feedforward connectivity in low gamma frequencies was significantly increased during the following 50 min nitrous oxide sedation (A, B). Frontoparietal connectivity in feedback and feedforward directions was increased during nitrous oxide induction in the mid gamma bandwidth (85–125 Hz) (C, D) whereas nitrous oxide sedation was characterized by a decrease in connectivity as compared to wakefulness. During post-nitrous oxide recovery period, connectivity in the feedback direction showed a modest increase relative to baseline wakefulness, while feedforward connectivity did not statistically differ from wake. Nitrous oxide induction transiently increased the strength of connectivity between frontal and parietal cortices in the high gamma bandwidth (125–175 Hz) in feedback and feedforward directions (E, F) whereas the nitrous oxide sedation phase was characterized by decrease in both feedback and feedforward connectivity to wake levels. Frontoparietal connectivity did not differ between baseline wakefulness and post-nitrous oxide recovery. A linear mixed model with a random intercept for each rat was used for statistical comparisons. Post hoc pairwise tests between states were performed with single-step correction for multiple comparisons via Tukey’s Test. The box plots show the median (horizontal bar) and interquartile range for averaged data over all 12 subjects at each epoch. The whiskers represent the minimum and maximum values within each epoch. The data for each subject are displayed by colored dots, with each color corresponding to a single subject across all epochs. NI – nitrous oxide induction, NS – nitrous oxide sedation, NSTE – normalized symbolic transfer entropy, PN – post-nitrous oxide, W – wake. *Significant compared to wake, #significant compared to nitrous oxide induction, §significant compared to nitrous oxide sedation. The statistical comparisons are shown at P < .05. The exact P values are provided in the text in the results section. The mean, SD, and F-statistics for statistical comparisons are provided in Supplemental Digital Content, Table 6.
In the mid gamma bandwidth, nitrous oxide induction was characterized by increase in bidirectional frontoparietal connectivity [feedback: t(141)=6.43, P < .001, Fig 6C; feedforward: t(141) = 6.09, P < .001, Fig. 6D]. During nitrous sedation, mid gamma connectivity did not significantly differ from wake, but was significantly lower than nitrous induction [feedback: t(141)=−7.15, P < .001, Fig. 6C; feedforward: t(141)=−7.72, P < .001, Fig. 6D]. As compared to the wake state, the post-nitrous period was characterized by a significant attenuation of mid gamma feedback connectivity [t(141)=−2.69, P = .04, Fig. 6C], while there was no significant effect on feedforward connectivity [t(141)=−2.50, P = .06, Fig. 6D].
High gamma frontoparietal connectivity during nitrous oxide induction increased in feedback [t(141)=3.90, P < .001, Fig. 6E] and feedforward [t(141)=4.52, P < .001, Fig. 6F] directions. During nitrous oxide sedation, high gamma connectivity returned to the baseline wake state but was significantly attenuated as compared to that observed during nitrous oxide induction [feedback: t(141)=−4.19, P < .001, Fig. 6E; feedforward: t(141)=−4.02, P < .001, Fig. 6F]. Frontoparietal connectivity during post-nitrous oxide recovery wakefulness did not statistically differ from the baseline wake state [feedback: t(141)=−0.35, P = 1, Fig. 6E; feedforward: t(141)=−0.24, P = 1, Fig. 6F], and was significantly attenuated when compared with nitrous oxide induction [feedback: t(141)=−4.126, P < .001, Fig. 6E; feedforward: t(141)=−4.673, P < .001, Fig. 6F]. Correlations between frontoparietal connectivity in gamma bandwidths and acetylcholine levels in prefrontal and parietal cortices are reported in Supplemental Digital Content, Table 8.
Discussion
The principal findings in our study demonstrate that changes in temporospatial EEG complexity during subanesthetic ketamine or 50% nitrous oxide exposure correlate with concomitant changes in prefrontal and parietal acetylcholine levels. Of note, the changes observed in EEG complexity showed distinct drug-dependent temporal and spatial profiles. Subanesthetic ketamine produced increases in signal complexity and cortical acetylcholine that lasted throughout the ketamine infusion (62.5 min), with the largest complexity changes occurring in frontal, posterior parietal, and occipital channel clusters. Conversely, nitrous oxide increased EEG complexity and cortical acetylcholine only during the first 12.5 min of nitrous administration (induction), with the largest magnitude of change in frontotemporal and parietal regions. The next 50 min of nitrous oxide exposure (sedation) were marked by periodically quiescent behavior, gradual decline in prefrontal and parietal acetylcholine levels, and global suppression of EEG complexity that was most pronounced in frontal channel clusters. The topographic distribution of changes in EEG complexity during subanesthetic ketamine and nitrous oxide sedation in our study broadly mirrored recent magnetoencephalographic and EEG data published during ketamine and nitrous oxide administration in humans,12,28 suggesting a comparable dynamic landscape during the administration of dissociative anesthetics in rodents.
We have shown earlier that ketamine, in the absence of any behavioral arousal, can increase prefrontal acetylcholine.19 A previous study quantifying the effect of nitrous oxide on cortical acetylcholine showed similar effects on acetylcholine as reported by us in the current study, i.e., a sharp increase after nitrous administration followed by a progressive decrease.20 Cortical acetylcholine is also known to correlate with behavioral arousal.17 Therefore, changes in cortical acetylcholine can result from a direct drug effect or changes in level of behavioral arousal. In the current study, it is difficult to ascribe the increase in cortical acetylcholine only to drug (ketamine/nitrous oxide) administration or only behavioral effects; it is likely that changes in cortical acetylcholine are a product of interaction between drug and behavioral effects. In contrast, increase in complexity is likely due to increased cortical acetylcholine or EEG activation, rather than behavioral arousal. This conclusion is supported by our recent studies in which we showed that a pharmacologically induced increase in prefrontal acetylcholine in sevoflurane-anesthetized rats was accompanied with EEG activation and an increase in complexity to baseline wake levels, with or without concomitant behavioral arousal.24,25
In addition to the relationship between neurophysiologic complexity and cortical acetylcholine, our study characterized changes in directed frontoparietal connectivity in low (25–55 Hz), mid (85–125 Hz), and high (125–175 Hz) gamma bandwidths. A previous study from our laboratory demonstrated that frontoparietal connectivity in high gamma bandwidth is a correlate of wakefulness, being reduced in conjunction with suppressed cortical levels of acetylcholine during general anesthesia.21 Our data extend these findings to dynamics characteristic of psychedelic states, demonstrating that periods of elevated cortical acetylcholine and neurophysiologic complexity during subanesthetic ketamine or nitrous oxide induction were correlated with increased high gamma frontoparietal connectivity. Conversely, low gamma frontoparietal connectivity was reduced when cortical acetylcholine levels were high and increased when acetylcholine levels were suppressed. Our data relating to cortical complexity and connectivity are broadly supportive of studies demonstrating hyperfrontality during classical psychedelic and dissociative drug administration, wherein excessive glutamatergic or cholinergic tone in frontal cortex accompanies aberrant cortical dynamics and alterations to the contents of consciousness.29–31
Reductions in measures of neurophysiologic complexity have been demonstrated across a broad range of pharmacologic and non-pharmacologic models of unconsciousness,6–10,24,32,33 while an increase in neurophysiologic complexity during psychedelic states has been shown to correlate with subjective intensity of altered conscious contents.12,13 Our finding of concomitant changes in cortical acetylcholine levels, neurophysiologic complexity, and high gamma frontoparietal connectivity during elevated and depressed states of consciousness is consistent with the purported role of acetylcholine in supporting cortical activation and arousal, expanding this relationship to dynamic neurophysiologic signatures related to the level of consciousness.3–5 Heterogeneous cell populations within the basal forebrain drive acetylcholine release at topographically specific targets across the cortex,14,34 with cortical cholinergic transmission shaping the activity of pyramidal and GABAergic interneuron populations implicated in cortical activation and wakefulness.35–37Acetylcholine release within the cortex is thought to create conditions that are permissive of high levels of neurophysiologic complexity, such as the reduction of slow cortical oscillations,38 thalamocortical desynchronization,39 and the promotion of high-frequency cortical gamma oscillations.19,35,40 Thus, while further testing of this hypothesis is required, cortical cholinergic transmission is a plausible neuromodulatory mechanism by which dynamics such as complexity can be shaped within cortical networks.
Although an increase in cortical acetylcholine levels during subanesthetic ketamine and nitrous oxide in rats has been reported, there are key differences as compared to our study. First, to our knowledge, none of the previous studies recorded simultaneous changes in EEG, which in the current study has allowed us to bridge neurochemical events in the cortex with concurrent changes in neurophysiological complexity and corticocortical connectivity. Second, while previous studies in rodents have reported changes in complexity across altered states of arousal,6,10,24 our use of high-density EEG allowed the application of temporospatial measures of complexity that more readily translate to those used in human studies.7,8,12 Third, because of our carefully titrated and timed drug administration, we were able to clearly characterize neurochemical and neurophysiological changes before, during, and after drug administration. Of note, although there are known sex differences in sensitivity and response to anesthetics, we did not find any overt behavioral differences between male and female rats. The lack of overt behavioral differences could be due to the graded nature of subanesthetic state that encompasses a wide range of behaviors, unlike anesthetic induction or emergence, which have binary endpoints (i.e., awake or anesthetized).
There are limitations to our study. Given the low temporal resolution of microdialysis, our results should only be interpreted as they relate to sustained tonic acetylcholine release. Alternative approaches such as cholinergic biosensors may be better suited to investigate phasic cholinergic dynamics as they relate to the neurophysiologic complexity at finer scales such as spikes or event-related potentials. Although cortical acetylcholine appears to vary with the level of consciousness, neurophysiologic complexity is likely to be governed by a complex neurochemical milieu, and thus further research investigating the relationship between complexity and other neurotransmitter systems within the cortex is warranted. We did not study complexity and cortical acetylcholine at a higher ketamine concentration sufficient to produce sedation or anesthesia, which could have provided insightful comparisons with the changes in these outcome measures during the sedation phase produced by nitrous oxide. However, in a previous study, we did demonstrate that neurophysiologic complexity is reduced during the state of anesthesia induced by intraperitoneal ketamine, demonstrating a relationship between state and complexity.10 Interestingly, while subanesthetic ketamine in human volunteers increases neurophysiologic complexity,9,12 anesthetic doses of ketamine have been shown to produce an alternating pattern of high and low complexity states.9 Finally, our data are correlative, and thus further studies establishing a causal relationship between cholinergic neurotransmission and complexity are necessary.
In conclusion, we demonstrate that changes in cortical acetylcholine are correlated with measures of EEG complexity and frontoparietal connectivity in high-gamma bandwidths during subanesthetic ketamine and nitrous oxide. These findings expand our understanding of the relationship between neurochemical and neurophysiologic signatures purported to correlate with consciousness.
Supplementary Material
Supplemental Digital Content, Figure 1: Schematic showing the EEG montage (30 screw electrodes) to record high-density intracranial monopolar EEG, and placement of microdialysis probes in prefrontal cortex and somatosensory barrel field region of the parietal cortex. A single screw electrode on the nasal sinus served as a reference while another electrode over the cerebellum served as the ground.
Supplemental Digital Content, Figure 2: Histological verification of microdialysis probe placement in prefrontal and parietal cortices. Stereotaxic maps from the rat brain atlas (Paxinos and Watson, 2006) show the location of microdialysis probes in the prelimbic (PrL) region of prefrontal cortex and somatosensory barrel field (S1BF) region of parietal cortex. Pink cylinders represent probes from ketamine experiments while purple cylinders represent probes from nitrous oxide experiments (A, C). B and D show cresyl violet-stained representative coronal brain sections (40 μm) through prefrontal cortex and parietal cortex, respectively. fmi – forceps minor corpus callosum, IL – infralimbic area, L – lateral parietal association cortex, M – medial parietal association cortex. The numbers on top right of the stereotaxic maps show the distance from Bregma; positive numbers are anterior to Bregma while negative numbers are posterior to Bregma.
Supplemental Digital Content, Figure 3: Changes in normalized power before, during, and after subanesthetic ketamine or nitrous oxide administration. Subanesthetic ketamine infusion (K) induced sustained increases in theta power (4–10 Hz) compared to baseline wakefulness (W), while theta power during nitrous oxide exposure did not significantly differ from wake state at either induction (NI) or sedation (NS) periods (A, B). Subanesthetic ketamine infusion induced sustained increases in low gamma power (25–55 Hz), increasing progressively as a function of the length of the infusion (C). Nitrous oxide in the induction period did not produce statistically significant change in low gamma power as compared to baseline wake state but attenuated low gamma power during the following 50 minutes of sedation (D). In the mid gamma bandwidth (85–125 Hz), subanesthetic ketamine was found to enhance power for the entirety of the infusion period as compared to that observed during baseline wake state (E). In contrast, nitrous oxide produced state-dependent effects on mid gamma power when compared with wakefulness, enhancing mid gamma power during the induction period, while significantly attenuating compared it during sedation period (F). Subanesthetic ketamine infusion promoted a progressive increase in power in the high gamma bandwidth (125–175 Hz) as compared to baseline wake state (G), while nitrous oxide was found to exert state-dependent effects – promoting high gamma power during induction period as compared to baseline wake state while suppressing it during sedation period (H). The statistical comparisons are shown at P < .05. K – subanesthetic ketamine infusion, NI – nitrous oxide induction, NS – nitrous oxide sedation, PK – post-ketamine recovery, PN – post-nitrous oxide, W – wake. *Significant compared to wake, #significant compared to subanesthetic ketamine infusion or nitrous oxide induction, §significant compared to nitrous oxide sedation.
Supplemental Digital Content, Table 1 Cortical acetylcholine before, during, and after intravenous infusion of subanesthetic ketamine.
Supplemental Digital Content, Table 2 Cortical acetylcholine before, during, and after nitrous oxide administration.
Supplemental Digital Content, Table 3 Normalized Lempel-Ziv complexity before, during, and after intravenous infusion of subanesthetic ketamine.
Supplemental Digital Content, Table 4 Normalized Lempel-Ziv complexity before, during, and after nitrous oxide administration.
Supplemental Digital Content, Table 5 Directed frontoparietal connectivity in gamma bandwidths before, during, and after intravenous infusion of subanesthetic ketamine.
Supplemental Digital Content, Table 6 Directed frontoparietal connectivity in gamma bandwidths before, during, and after nitrous oxide administration.
Supplemental Digital Content, Table 7 Correlation between changes in cortical acetylcholine and directed frontoparietal connectivity in gamma bandwidths after intravenous infusion of subanesthetic ketamine.
Supplemental Digital Content, Table 8 Correlation between changes in cortical acetylcholine and directed frontoparietal connectivity in gamma bandwidths in the nitrous oxide group.
Question:
What is the relationship between neurophysiologic complexity and cortical acetylcholine?
Findings:
Subanesthetic ketamine- and nitrous oxide-induced changes in cortical acetylcholine correlated with concurrent changes in neurophysiologic complexity, connectivity, and behavior.
Meaning:
The relationship between cortical acetylcholine and neurophysiologic complexity prompts further investigation of subcortical cholinergic nuclei in the modulation of cortical neurophysiologic complexity.
Acknowledgements:
We thank Chris Andrews Ph.D (CSCAR, the University of Michigan, 915 E. Washington St. Ann Arbor, MI 48109-1070) for consultation and assistance with statistical analysis.
This work was supported by the National Institutes of Health (grant no. R01GM111293), the National Science Foundation Graduate Research Fellowship (grant no. DGE 1256260), the Center for Consciousness Science, and the Department of Anesthesiology, University of Michigan.
Glossary
- ACh
acetylcholine
- EEG
electroencephalogram
- K
subanesthetic ketamine infusion
- LZs
Lempel-Ziv complexity
- LZsN
normalized Lempel-Ziv complexity
- NI
nitrous oxide induction
- NMDA
N-Methyl-D-aspartate
- NS
nitrous oxide sedation
- NSTE
normalized symbolic transfer entropy
- PK
post-ketamine recovery
- PN
post-nitrous oxide recovery
- W
wake
Footnotes
Conflicts of Interests/Financial Disclosures: NONE
References
- 1.Boly M et al. Are the Neural Correlates of Consciousness in the Front or in the Back of the Cerebral Cortex? Clinical and Neuroimaging Evidence. J. Neurosci 37, 9603–9613 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odegaard B, Knight RT & Lau H Should a Few Null Findings Falsify Prefrontal Theories of Conscious Perception? J. Neurosci 37, 9593–9602 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tononi G, Boly M, Massimini M & Koch C Integrated information theory: from consciousness to its physical substrate. Nat. Rev. Neurosci 17, 450–461 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Carhart-Harris RL The entropic brain - revisited. Neuropharmacology 142, 167–178 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Casali AG et al. A Theoretically Based Index of Consciousness Independent of Sensory Processing and Behavior. Sci. Transl. Med 5, 198ra105–198ra105 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Abásolo D, Simons S, Morgado da Silva R, Tononi G & Vyazovskiy VV Lempel-Ziv complexity of cortical activity during sleep and waking in rats. J. Neurophysiol 113, 2742–2752 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schartner M et al. Complexity of Multi-Dimensional Spontaneous EEG Decreases during Propofol Induced General Anaesthesia. PLOS ONE 10, e0133532 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schartner MM et al. Global and local complexity of intracranial EEG decreases during NREM sleep. Neurosci. Conscious niw022 (2017) doi: 10.1093/nc/niw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li D & Mashour GA Cortical dynamics during psychedelic and anesthetized states induced by ketamine. NeuroImage 196, 32–40 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brito MA, Li D, Mashour GA & Pal D State-Dependent and Bandwidth-Specific Effects of Ketamine and Propofol on Electroencephalographic Complexity in Rats. Front. Syst. Neurosci 14, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tagliazucchi E, Carhart-Harris R, Leech R, Nutt D & Chialvo DR Enhanced repertoire of brain dynamical states during the psychedelic experience: Enhanced Repertoire of Brain Dynamical States. Hum. Brain Mapp 35, 5442–5456 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schartner MM, Carhart-Harris RL, Barrett AB, Seth AK & Muthukumaraswamy SD Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Sci. Rep 7, 46421 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timmermann C et al. Neural correlates of the DMT experience assessed with multivariate EEG. Sci. Rep 9, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Záborszky L et al. Neurons in the Basal Forebrain Project to the Cortex in a Complex Topographic Organization that Reflects Corticocortical Connectivity Patterns: An Experimental Study Based on Retrograde Tracing and 3D Reconstruction. Cereb. Cortex 25, 118–137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrosu F et al. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 671, 329–332 (1995). [DOI] [PubMed] [Google Scholar]
- 16.Nelson CL, Burk JA, Bruno JP & Sarter M Effects of acute and repeated systemic administration of ketamine on prefrontal acetylcholine release and sustained attention performance in rats. Psychopharmacology (Berl.) 161, 168–179 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Lydic R & Baghdoyan HA Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology 103, 1268–1295 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Nair SG & Gudelsky GA Activation of 5-HT2 receptors enhances the release of acetylcholine in the prefrontal cortex and hippocampus of the rat. Synapse 53, 202–207 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Pal D, Hambrecht-Wiedbusch VS, Silverstein BH & Mashour GA Electroencephalographic coherence and cortical acetylcholine during ketamine-induced unconsciousness. Br. J. Anaesth 114, 979–989 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shichino T et al. Effects of inhalation anaesthetics on the release of acetylcholine in the rat cerebral cortex in vivo. Br. J. Anaesth 80, 365–370 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Pal D, Silverstein BH, Lee H & Mashour GA Neural Correlates of Wakefulness, Sleep, and General Anesthesia: An Experimental Study in Rat. Anesthesiology 125, 929–942 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lempel A & Ziv J On the Complexity of Finite Sequences. IEEE Trans. Inf. Theory 22, 75–81 (1976). [Google Scholar]
- 23.Lee U et al. Disruption of Frontal–Parietal Communication by Ketamine, Propofol, and Sevoflurane. Anesthesiology 118, 1264–1275 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pal D et al. Level of Consciousness Is Dissociable from Electroencephalographic Measures of Cortical Connectivity, Slow Oscillations, and Complexity. J. Neurosci 40, 605–618 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pal D et al. Differential Role of Prefrontal and Parietal Cortices in Controlling Level of Consciousness. Curr. Biol 28, 2145–2152.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. (2016). [Google Scholar]
- 27.Lorenz DJ, Datta S & Harkema SJ Marginal association measures for clustered data. Stat. Med 30, 3181–3191 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vrijdag XCE, van Waart H, Mitchell SJ & Sleigh JW An Electroencephalogram Metric of Temporal Complexity Tracks Psychometric Impairment Caused by Low-dose Nitrous Oxide. Anesthesiology 134, 202–218 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Vollenweider FX et al. Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F]fluorodeoxyglucose (FDG). Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol 7, 9–24 (1997). [DOI] [PubMed] [Google Scholar]
- 30.Abdallah CG et al. The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology 43, 2154–2160 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mason NL et al. Me, myself, bye: regional alterations in glutamate and the experience of ego dissolution with psilocybin. Neuropsychopharmacology 45, 2003–2011 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hudetz AG, Liu X, Pillay S, Boly M & Tononi G Propofol anesthesia reduces Lempel-Ziv complexity of spontaneous brain activity in rats. Neurosci. Lett 628, 132–135 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bodart O et al. Measures of metabolism and complexity in the brain of patients with disorders of consciousness. NeuroImage Clin. 14, 354–362 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benarroch EE Acetylcholine in the cerebral cortex: Effects and clinical implications. Neurology 75, 659–665 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Liljenstrom H & Hasselmo ME Cholinergic modulation of cortical oscillatory dynamics. J. Neurophysiol 74, 288–297 (1995). [DOI] [PubMed] [Google Scholar]
- 36.Poulet JFA & Crochet S The Cortical States of Wakefulness. Front. Syst. Neurosci 12, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yi F et al. Direct excitation of parvalbumin-positive interneurons by M1 muscarinic acetylcholine receptors: roles in cellular excitability, inhibitory transmission and cognition. J. Physiol 592, 3463–3494 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L et al. Basal Forebrain Cholinergic Neurons Primarily Contribute to Inhibition of Electroencephalogram Delta Activity, Rather Than Inducing Behavioral Wakefulness in Mice. Neuropsychopharmacology 41, 2133–2146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colangelo C, Shichkova P, Keller D, Markram H & Ramaswamy S Cellular, Synaptic and Network Effects of Acetylcholine in the Neocortex. Front. Neural Circuits 13, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howe WM et al. Acetylcholine Release in Prefrontal Cortex Promotes Gamma Oscillations and Theta–Gamma Coupling during Cue Detection. J. Neurosci 37, 3215–3230 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content, Figure 1: Schematic showing the EEG montage (30 screw electrodes) to record high-density intracranial monopolar EEG, and placement of microdialysis probes in prefrontal cortex and somatosensory barrel field region of the parietal cortex. A single screw electrode on the nasal sinus served as a reference while another electrode over the cerebellum served as the ground.
Supplemental Digital Content, Figure 2: Histological verification of microdialysis probe placement in prefrontal and parietal cortices. Stereotaxic maps from the rat brain atlas (Paxinos and Watson, 2006) show the location of microdialysis probes in the prelimbic (PrL) region of prefrontal cortex and somatosensory barrel field (S1BF) region of parietal cortex. Pink cylinders represent probes from ketamine experiments while purple cylinders represent probes from nitrous oxide experiments (A, C). B and D show cresyl violet-stained representative coronal brain sections (40 μm) through prefrontal cortex and parietal cortex, respectively. fmi – forceps minor corpus callosum, IL – infralimbic area, L – lateral parietal association cortex, M – medial parietal association cortex. The numbers on top right of the stereotaxic maps show the distance from Bregma; positive numbers are anterior to Bregma while negative numbers are posterior to Bregma.
Supplemental Digital Content, Figure 3: Changes in normalized power before, during, and after subanesthetic ketamine or nitrous oxide administration. Subanesthetic ketamine infusion (K) induced sustained increases in theta power (4–10 Hz) compared to baseline wakefulness (W), while theta power during nitrous oxide exposure did not significantly differ from wake state at either induction (NI) or sedation (NS) periods (A, B). Subanesthetic ketamine infusion induced sustained increases in low gamma power (25–55 Hz), increasing progressively as a function of the length of the infusion (C). Nitrous oxide in the induction period did not produce statistically significant change in low gamma power as compared to baseline wake state but attenuated low gamma power during the following 50 minutes of sedation (D). In the mid gamma bandwidth (85–125 Hz), subanesthetic ketamine was found to enhance power for the entirety of the infusion period as compared to that observed during baseline wake state (E). In contrast, nitrous oxide produced state-dependent effects on mid gamma power when compared with wakefulness, enhancing mid gamma power during the induction period, while significantly attenuating compared it during sedation period (F). Subanesthetic ketamine infusion promoted a progressive increase in power in the high gamma bandwidth (125–175 Hz) as compared to baseline wake state (G), while nitrous oxide was found to exert state-dependent effects – promoting high gamma power during induction period as compared to baseline wake state while suppressing it during sedation period (H). The statistical comparisons are shown at P < .05. K – subanesthetic ketamine infusion, NI – nitrous oxide induction, NS – nitrous oxide sedation, PK – post-ketamine recovery, PN – post-nitrous oxide, W – wake. *Significant compared to wake, #significant compared to subanesthetic ketamine infusion or nitrous oxide induction, §significant compared to nitrous oxide sedation.
Supplemental Digital Content, Table 1 Cortical acetylcholine before, during, and after intravenous infusion of subanesthetic ketamine.
Supplemental Digital Content, Table 2 Cortical acetylcholine before, during, and after nitrous oxide administration.
Supplemental Digital Content, Table 3 Normalized Lempel-Ziv complexity before, during, and after intravenous infusion of subanesthetic ketamine.
Supplemental Digital Content, Table 4 Normalized Lempel-Ziv complexity before, during, and after nitrous oxide administration.
Supplemental Digital Content, Table 5 Directed frontoparietal connectivity in gamma bandwidths before, during, and after intravenous infusion of subanesthetic ketamine.
Supplemental Digital Content, Table 6 Directed frontoparietal connectivity in gamma bandwidths before, during, and after nitrous oxide administration.
Supplemental Digital Content, Table 7 Correlation between changes in cortical acetylcholine and directed frontoparietal connectivity in gamma bandwidths after intravenous infusion of subanesthetic ketamine.
Supplemental Digital Content, Table 8 Correlation between changes in cortical acetylcholine and directed frontoparietal connectivity in gamma bandwidths in the nitrous oxide group.


