Fig. (2).
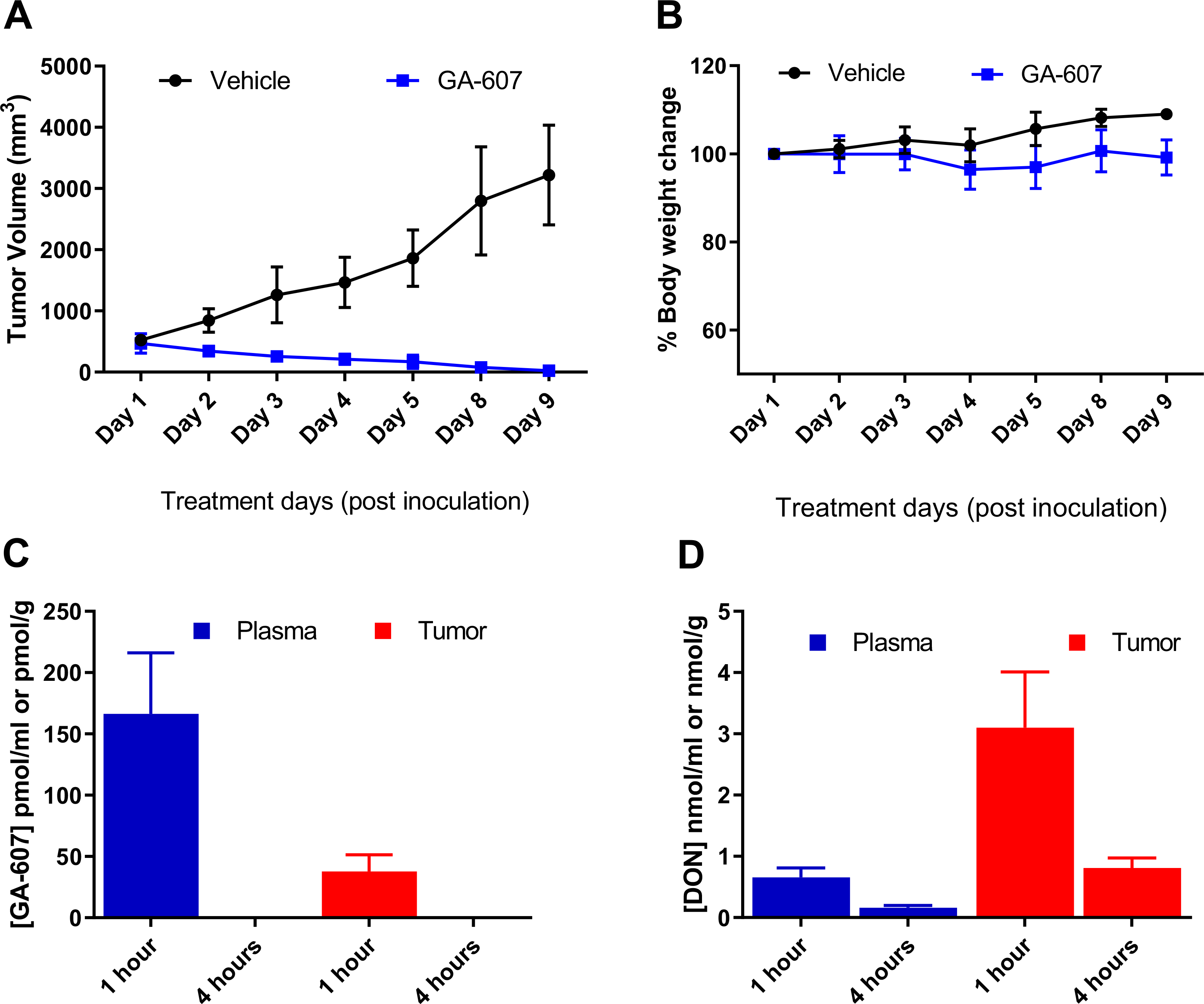
Efficacy, tolerability, and pharmacokinetic assessment of GA-607 in EL4 tumor-bearing mice following GA-607 (3.2 mg/kg SC) treatment; dosed 5 consecutive days followed by 2 drug-free days. Tumors volumes and body weights were only measured on the day of dosing. (A) Complete tumor regression was observed following GA-607 administration. (B) No change in body weight was observed following GA-607 administration. (C) GA-607 and (D) GA-607-derived DON levels in plasma and tumors following GA-607 administration.
