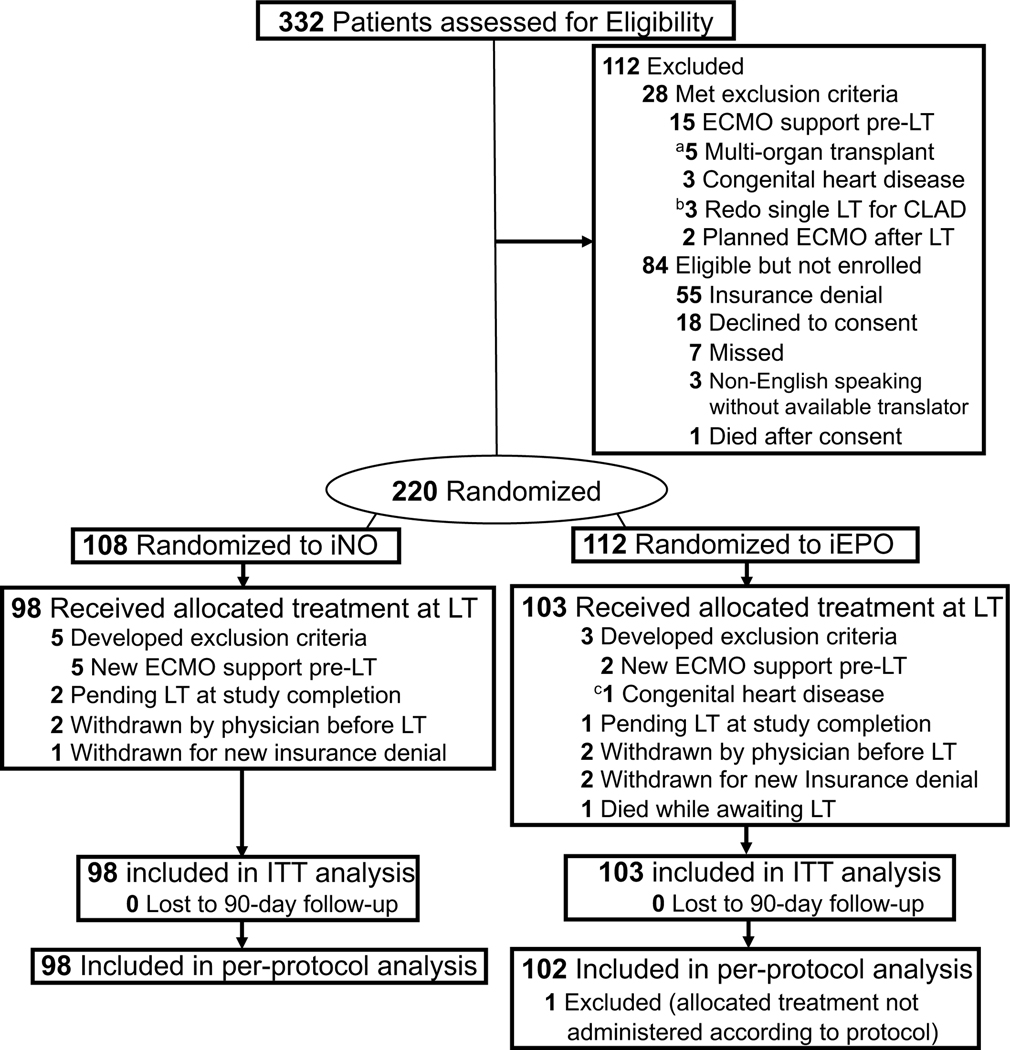
Figure 1. Flow of Participants in a Study of Inhaled Pulmonary Vasodilators for Adult Lung Transplantation.
In all analyses, patients were analyzed according to their randomized group. Participants were excluded from the intention-to-treat analysis if they were withdrawn, developed exclusion criteria after randomization, or remained on the LT list and were not transplanted. Those that received the allocated treatment at the time of LT were included in an intention-to-treat analysis. Study enrollment was completed once sample-size was achieved. None of the participants were lost to 90-day follow-up.
aMultiple organ transplantation included 1 patient for lung-kidney and 4 patients for lung-liver.
bPatient with diagnosis that did not fit one of the five randomization strata
cIneligible for enrollment, consented and randomized, then ineligibility was noted before LT.
CLAD, Chronic lung allograft dysfunction; ITT, Intention-to-treat; LT, Lung transplantation