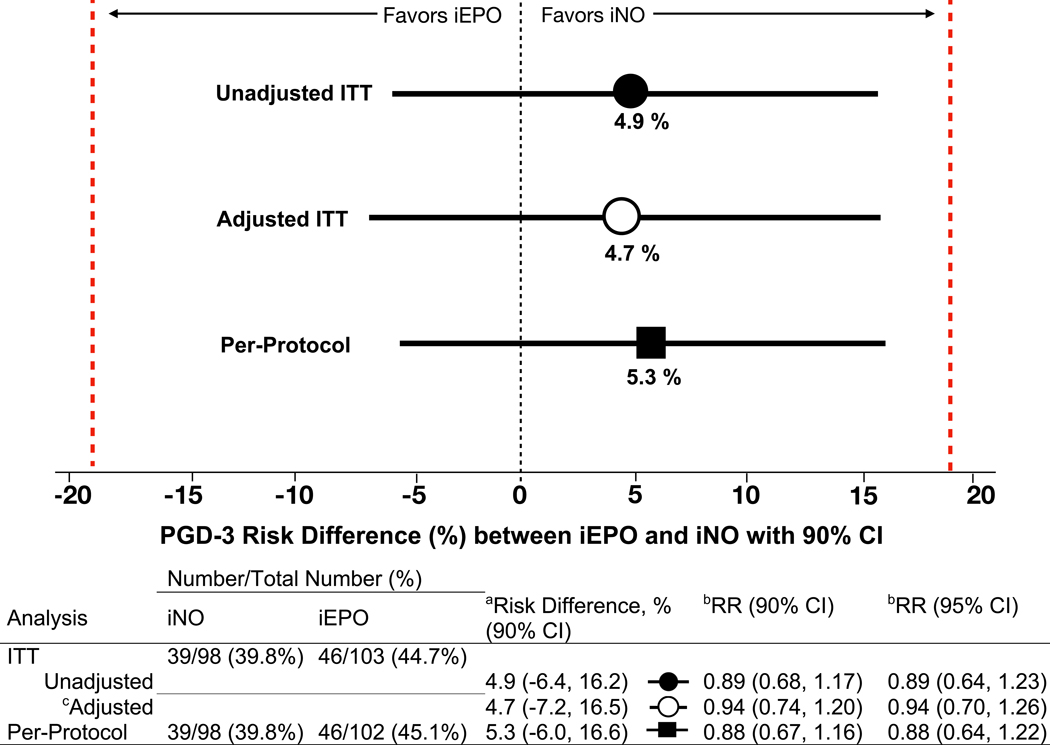
Figure 2. Risk differences and relative risks of PGD-3 development between iNO and iEPO treatment groups.
To determine the presence of clinical equivalence between iNO and iEPO, a lower and upper bounds of −19% and +19% was prespecified (red lines).
aRisk difference is the absolute difference between PGD-3 rates between groups and is determined by the two one-sided test (TOST) procedure. Setting α at 0.05 and testing the upper and lower bounds separately, equivalence is concluded only if both tests are significant. To transform this procedure into a single confidence interval, 1–2α (90%) is used and the TOST CI becomes the intersection of the two one-sided confidence intervals. A more conservative 95% CI (1-α) was determined and also demonstrated exclusion of the lower and upper bounds of the margin in support of equivalence for the unadjusted ITT (−8.6%, +18.3%), adjusted ITT (−9.5%, +18.8%) and per-protocol (−8.2%, +18.8%) analyses.
bRelative risk, RR, is the risk of developing PGD-3 if treated with iNO compared with iEPO.
cMultivariable logistic regression adjusting for delayed chest closure and donor-recipient sex mismatch from the selected model (Supplement 3, eTable 1). Risk difference and RR are derived from the multivariable logistic regression model. Differences between adjusted and unadjusted risk difference and RR are due to the difference in comparing two patients in the adjusted analysis with the same sex-mismatch and chest closure status. However, number of events and their distribution between the unadjusted and adjusted analyses remain the same.
CI, Confidence interval; iEPO, Inhaled epoprostenol; iNO, Inhaled nitric oxide; ITT, Intention-to-treat.