Figure 5:
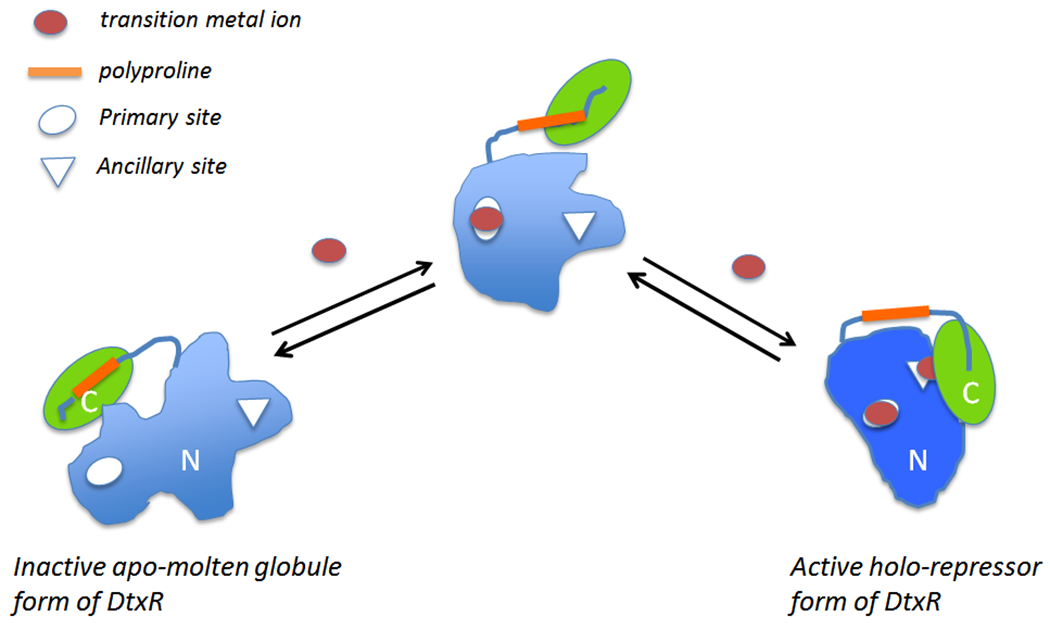
DtxR is a two-domain protein that contains two structurally and functional distinct transition metal ion binding sites. An activating transition metal ion first binds to the Primary site which orients the DNA binding helices and begins to fold the N-terminal domain. Subsequent binding of a metal ion to the Ancillary site reorients the folding of the SH3-like C-terminal domain and completes the formation of dimer interface of the holo-repressor (modified from Rangachari et al., 2005 (66)).
