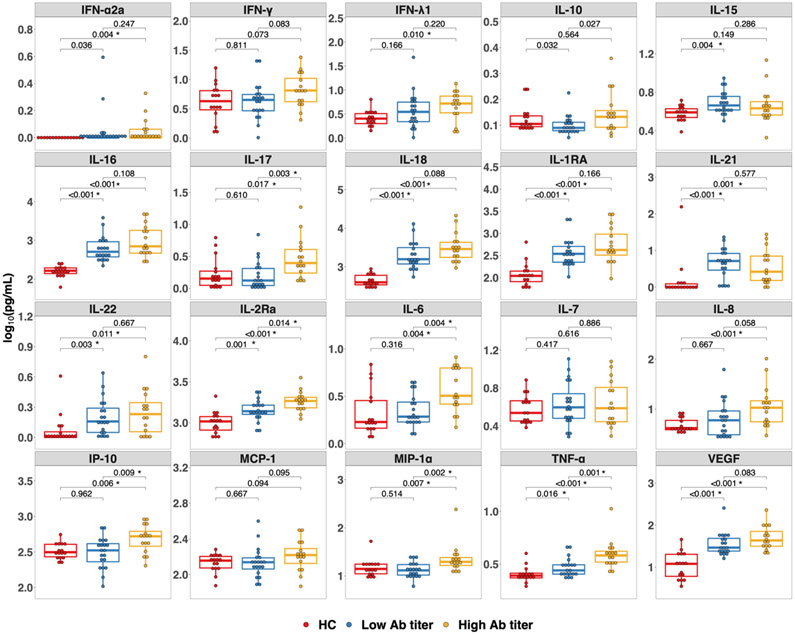
Figure 2.
Cytokines in solid organ transplant recipients (SOTRs) prior to a third dose of SARS-CoV-2 vaccine and healthy controls prior to first dose of a two-dose mRNA-based vaccine series. Each cytokine or chemokine measured is indicated in the grey header above each panel. Differences among the healthy controls (red, n = 16), SOTRs who developed low-titer responses (blue, n = 19), and SOTRs who developed high-titer responses (yellow, n = 16) after a third dose of SARS-CoV-2 vaccine were determined by a two-tailed Wilcoxon-Rank-Sum test. Multiple comparison was controlled using the Benjamini-Hochberg procedure with false discovery rate of 0.05. Significant P-values after adjusting for multiple comparison are marked with *. Low-titer (ratio < 3.5) and High-titer (ratio ≥ 3.5) were based on the classification of low-titer versus high-titer convalescent plasma set by the FDA.20 Ab, antibody; HC, healthy control; IFN, interferon; IL, interleukin; IP-10, Interferon-γ-inducible protein 10; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.