Figure 7. Therapeutic activity of MTL-CEBPA in combination with checkpoint inhibitors.
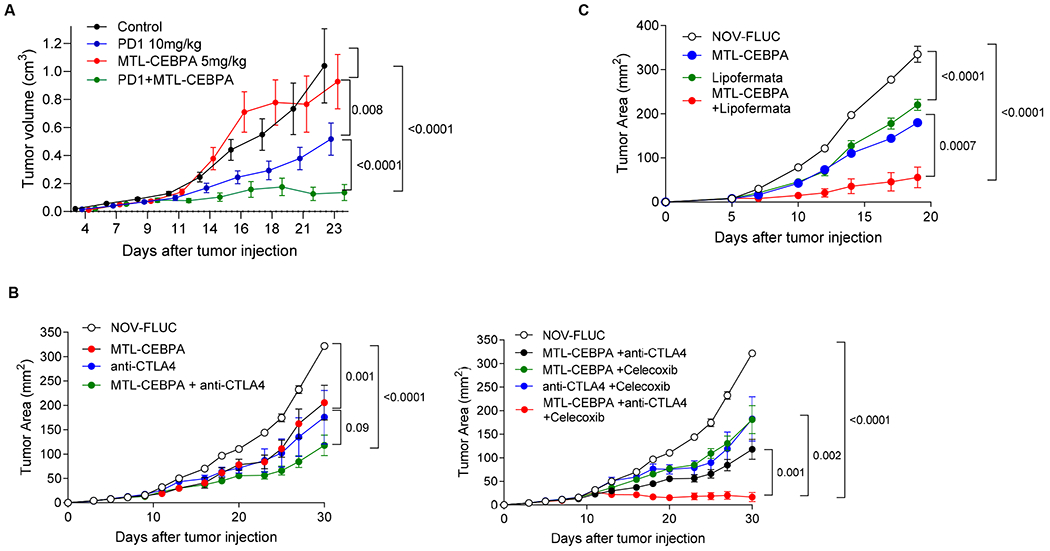
A. MC38 tumor-bearing mice were treated with MTL-CEBPA or NOV-FLUC control at 5 mg/kg from Day 4 (twice a week). Anti-PD1 antibody was intraperitoneally injected to the mice twice a week at 10 mg/kg. n=5 per group. Mean and SEM are shown. P values were calculated using two-way ANOVA test. B. LLC tumor-bearing mice were treated with MTL-CEBPA or NOV-FLUC control at 3 mg/kg from Day 3 (twice a week). Anti-CTLA4 antibody was intraperitoneally injected to the mice on Days 10, 17 and 24 (100 μg/mouse). Celecoxib was orally given to the mice at 50 mg/kg from Day 3 (daily). Mean and SEM (n=4) are shown. P values were calculated using two-way ANOVA test. C. LLC tumor-bearing mice were treated with MTL-CEBPA or NOV-FLUC (3 mg/kg from Day 3, twice a week) in combination with lipofermata (2 mg/kg, twice per day from Day 3, subcutaneously). In each experiment p values were calculated in two-way ANOVA.
