Abstract
Two in every three Alzheimer’s disease diagnoses are females, calling attention to the need to understand sexual dimorphisms with aging and neurodegenerative disease progression. Dysfunction and damage to the vasculature with aging are strongly linked to Alzheimer’s disease. With aging there is an increase in stiffness of the large elastic arteries, and this stiffening is associated with cerebrovascular dysfunction and cognitive impairment. However, it is unclear how the deleterious effects of arterial stiffness may differ between females and males. While environmental, chromosomal, and sex hormone factors influence aging, there is evidence that the deficiency of estrogen post-menopause in females is a contributor to vascular aging and Alzheimer’s disease progression. The purpose of this mini review is to describe the recent developments in our understanding of sex differences in large artery stiffness, cerebrovascular dysfunction, and cognitive impairment, and their intricate relations. Furthermore, we will focus on the impact of the loss of estrogen post-menopause as a potential driving factor for these outcomes. Overall, a better understanding of how sex differences influence aging physiology is crucial to the prevention and treatment of neurodegenerative diseases.
Keywords: arterial stiffness, pulse pressure, endothelial cell, cerebrovascular, cognitive impairment, menopause, estrogen
1. Introduction
Advancing age is the biggest risk factor for late-onset Alzheimer’s disease (AD), suggesting that elements of the aging process initiate or contribute to AD. In the United States, two-thirds of patients with AD are females (1) and the progression from mild cognitive impairment to AD is quicker in females than males (2). However, the causes of the increased AD risk in females are not entirely clear. The contribution of the aging vascular system in AD onset and progression is supported by recent evidence (3). Therefore, sex differences in vascular aging represent a potential source of the greater AD risk in females.
A primary characteristic of vascular aging is the stiffening of the large elastic arteries. This age-related increase in arterial stiffness is related to cognitive impairment and AD, and it is hypothesized that cerebrovascular dysfunction links these phenomena (4). While arterial stiffness increases with age in both sexes, there is a stronger association between arterial stiffness and mortality in females compared with males (5). Less is known about sex differences in the relations between arterial stiffness and cerebrovascular dysfunction and cognitive impairment. Sexual dimorphisms in age-related arterial stiffening, and the consequences of this stiffness, may explain the sex differences in AD risk, and potentially identify the need for individualized treatment. The goal of this mini review is to highlight the importance of sex differences in vascular aging and the related onset of cerebrovascular dysfunction and AD. Importantly, we will identify the major gaps in knowledge remaining. The impact of sex differences in vascular aging affects a broad range of neurological diseases. Although this mini review focuses on AD, most of the underlying physiological processes discussed have implications for other neurological diseases.
2. Sex Hormones
Sex differences in AD risk are likely driven by sex hormones, genotype (XX vs. XY), and sociocultural factors. In particular, the low estrogen in post-menopausal females is a contributor to vascular dysfunction when compared to pre-menopausal female and/or their male counterparts. Estrogen stimulates genomic and nongenomic cell signaling cascades by activation of estrogen receptors (ER) α and β, and the G-protein coupled receptor, GPER1 (or GPR30) (6,7). These receptors are found on vascular cells as well as other cells in the brain (8,9). Progesterone and androgens also decrease with age, while follicle stimulating hormone and luteinizing hormone increase (10,11). In this review, we will specifically focus on the low estrogen state in post-menopausal females given the preponderance of evidence for its importance.
3. Large Artery stiffness
The stiffness of the large elastic arteries increases with age in both males and females; yet there are important sex differences in the causes and rate of progression of this stiffening. The term large arteries, or large elastic arteries, refers to the aorta and carotid arteries. These large arteries have a very distensible wall and a high content of elastin protein. At young ages, females tend to have more compliant large arteries compared with males, but this trend reverses in old age with older females generally having stiffer large arteries compared with males (5,12,13). These trends result in females experiencing a more rapid increase in arterial stiffness with aging than males, as found in humans (14) and rodents (15). This rapid period of increases in arterial stiffness occurs at ~55–75 years of age in human females, corresponding to the early post-menopausal period and the reduction of estrogen. Hormone replacement therapy with estradiol typically improves arterial stiffness in post-menopausal females (16). In summary, age-related increases in large artery stiffness are more rapid in females, likely due to declining estrogen post-menopause.
In general, the sources of age-related large artery stiffening are decreased elastin content, increased elastin fragmentation, increased collagen content and crosslinking, and increased vascular tone (17,18). However, most of these mechanisms were studied in males and little is known about the causes of increased arterial stiffness in females. In animal studies, females have age-related increases in large artery collagen content and advanced glycation end-products, contributing to collagen cross-linking (15,19). Estrogen decreases collagen deposition by cultured smooth muscle cells (20), and thus, post-menopausal females may suffer from a loss of the inhibitory actions of estrogens on arterial collagen production. In addition to differences in structural proteins, age-related arterial stiffening in females is caused by increases in arterial tone from a reduction in nitric oxide (NO) bioavailability (16). Interventions known to improve NO bioavailability also reduce stiffness in post-menopausal females, such as treatment with antioxidants (21) and endothelial NO synthase (eNOS) co-factor tetrahydrobiopterin (22). Furthermore, sympathetic nerve activity increases with age in females and has been related to large artery stiffness, potentially due to increased arterial tone or blood pressures (23,24). Lastly, signaling by smooth muscle mineralocorticoid receptors contributes to increased age-related aorta stiffening, but the mechanisms appear to be different between male and female mice (15). The causes of sex differences in large artery stiffness have been more thoroughly reviewed by Moreau and Hildreth (25) and DuPont et. al. (26).
4. Blood Flow and Pressure Pulsatility
As large artery stiffness increases, there is greater pulsatility of blood pressure and blow flow (27). At young ages, the large arteries are highly compliant and dampen the pulse of blood ejected from the heart. The cerebral vasculature is also protected from highly pulsatile pressure and flow due to a partial reflection of the pressure wave before it reaches the brain. This partial reflection of the reflected wave results from the mismatch of stiffness between the highly complaint aorta and the stiffer muscular arteries (27). As the aorta stiffens with age, there is less pressure wave reflection and a higher transmission of pulsatile energy to small arteries, arterioles, and capillaries in the brain (27). It is thought that the resulting increased pressure and flow pulsatility in the cerebral vasculature leads to damage and dysfunction (28). While young females have lower cerebral artery blood flow pulsatility compared with young males (29), this protection does not persist into old age. In fact, the rate of increase in middle cerebral artery blood flow pulsatility with aging is greater in females than in males (29), corresponding to the more rapid increase in large artery stiffness in aging females. Older females also have less pulsatile dampening between the carotid and cerebral arteries compared with older males, (30) further illustrating a higher transmission of pulsatile energy into the brain of older females. These findings suggest that the female brain at young ages is protected from high pulse pressures, but is exposed to a rapid increase, greater than males, in pulse pressure with aging.
5. Cerebrovascular Endothelial Dysfunction
The age-related increase in pulse pressure in the cerebral vasculature is thought to cause endothelial cell dysfunction. The endothelium is an integral regulator of cerebral blood flow and blood brain barrier (BBB) permeability, thus age-related dysfunction of the cerebral endothelium can lead to impairment in the brain. Endothelial cells react to stimuli by releasing several substances that cause dilation or constriction of blood vessels. At the arteriole and capillary level, a properly functioning endothelial layer is needed to coordinate the vascular, immune, and neural cells that comprise the neurovascular unit (31). A key function of endothelial cells is to produce NO that signals smooth muscle cells and pericytes for relaxation (32). During aging, decreased NO bioavailability is caused by increased oxidative stress, specifically via the reaction of superoxide with NO (33). This reduction in NO bioavailability with aging can lead to an imbalance of vasodilation and vasoconstriction signals and poses a major issue for the tight regulation of cerebral blood flow.
The BBB protects the brain from circulating pathogens and is composed of endothelial cells joined together by tight junction proteins (31). The health of endothelial cells, as well as other cells of the neurovascular unit, is important to maintaining a functional barrier. Furthermore, brain endothelial cells can tightly regulate transcytosis, limiting vesicle-mediated movement of solutes in and out of the brain (31). Dysfunction of the BBB contributes to AD by allowing the entrance of substances (e.g., neurotoxins, immune cells) that result in increased inflammatory signaling and oxidative stress, stimulating amyloid-β (Aβ) production (34). A dysfunctional BBB will also lead to impaired clearance of Aβ from the brain, and this impaired clearance is thought to be the primary cause of Aβ plaque deposition in AD (35). Thus, age-related dysfunction of endothelial cells contributes to impaired cerebral blood flow and a dysfunctional BBB.
Estrogen acts favorably on the cerebral vasculature by improving the function of endothelial cells (36), a phenomenon that is lost post-menopause. The endothelium has widely expressed ERα, and binding to this receptor results in increased eNOS expression and activation via phosphorylation, leading to greater endothelial dependent vasodilation (37). Estrogen also decreases oxidative stress by reducing mitochondrial superoxide production (38) and increasing endogenous antioxidants (39). In post-menopausal females, there is decreased ERα expression in the vasculature (40) and post-menopausal females have marked impairments in endothelial function compared with pre-menopausal females (41). See Robinson et. al. for a more thorough review of this topic (42)
The cerebral vasculature appears to be particularly susceptible to the damaging effects of increased large artery stiffness and pulse pressure. High pulse pressures applied to cerebral arteries ex vivo, as well as circumferential stress of cultured endothelial cells, leads to increased oxidative stress (43–45). Greater large artery stiffness in a rodent model leads to impaired cerebral artery endothelium-dependent vasodilation by increased oxidative stress and decreased NO bioavailability (46). Increased large artery stiffness also leads to a more permeable BBB in rodents (47). However, these mechanistic studies have yet to be performed in females. It is reasonable to assume that young females are doubly protected against this phenomenon owing to lower arterial stiffness and the protective effects of estrogens directly on the endothelium. The endothelium of older females may be more susceptible to the negative consequences of large artery stiffness, but this is an area that requires more investigation.
6. Cerebral Blood Flow
Cerebral endothelial cell dysfunction will disturb the tight regulation of blood flow in the brain. Young females have greater cerebral blood flow compared with males; however, the declines in cerebral blood flow with aging are greater in females, such that at old ages there are no differences in cerebral blood flow between females and males (48–50). More important than global cerebral blood flow is the ability for local blood flow to change in response to stimuli and to be directed to working regions of the brain, indicated by cerebrovascular reactivity. Cerebrovascular reactivity declines with advancing age to a greater extent in females than males, and hormone replacement therapy can preserve cerebrovascular reactivity in post-menopausal females (51). The sex differences in cerebral blood flow and reactivity with aging, as well as the mechanisms, are extensively reviewed in Barnes and Charkoudian (52).
While sex differences in cerebral blood flow and reactivity are extensively investigated, less is known about the relation of sex differences and large artery stiffness. The association between large artery stiffness and reduced cerebral blood flow or cerebrovascular reserve has been demonstrated in human subjects, but this was independent of sex (50) or was not analyzed for sex differences (53). Rodent models of induced large artery stiffness demonstrate the cause-and-effect relation between large artery stiffness and reduced cerebral perfusion (47,54) but these studies were performed in only male rodents. Thus, a crucial area for future research is to understand the impact of sex and sex hormones on the relation of large artery stiffness and cerebral blood flow regulation, as well as the potential modulation of this relation by other factors.
7. Neuropathology
Endothelial dysfunction, BBB permeability, and reduced cerebral blood flow are key mechanisms leading to other pathologies in the brain. For example, large artery stiffness is related to cerebral small vessel disease, a disease that is characterize by hyperintensities, cerebral microbleeds and lacunar infarcts (55–59). Aortic augmentation index, an indicator of arterial stiffness, is also related to white matter hyperintensities in post-menopausal females (60). However, no other studies have examined sex differences in the relation between cerebral small vessel disease and arterial stiffness.
Large artery stiffness is also related to lower brain volumes abnormalities and amyloid-β deposition (55,59). The causative nature of increased large artery stiffness on neurodegeneration and neuroinflammation was demonstrated in rodents (61). There is a suggestion that these relations between peripheral pulse pressure and neuropathology may have sex differences, as it was found that females had a stronger correlation between brachial pulse pressure and white matter microstructure changes (62). Notably, this strong correlation in females is only true early post-menopause and is not found for the group over 75 years of age (62), corresponding to the period of more rapid stiffening of the large arteries. Thus, studies indicate an association between large artery stiffness and neuropathology, but the knowledge of how sex and sex hormones effect these relations is very limited.
8. Cognitive function
Large artery stiffness, and the resultant cerebrovascular dysfunction, will potentially impact the brain, leading to cognitive impairment. The literature regarding sex differences in cognitive function in older adults is inconsistent. This is partly due to sex differences in the specific types of cognitive function that change with age. Older females typically score better on verbal tasks than males, while older males score better on visuospatial and motor coordination than females (63). An important sex difference is that older females experience a more rapid cognitive decline, with the transition from mild cognitive impairment to AD occurring faster compared with age-matched males (2). There are numerous studies demonstrating a correlation between greater large artery stiffness and cognitive impairment. While most of these studies controlled for sex in their analyses, none of them report analysis specifically for sex differences in these relations (59,64–68) except the study by Singer et al. In that study of subjects 70–90 years of age, a relation between large artery stiffness and memory was found in males, but not females (69). However, as the rapid progression of arterial stiffness occurs from 55–75 years of age in females, this study may have missed the key time for relations in females. In rodents, induced carotid stiffening leads to cognitive impairment, but these studies are limited to male rodents to date (47). The sex differences in the relation of arterial stiffness and cognitive decline are likely more complex than just differences in sex hormones. For example, history of pregnancy and childbirth may contribute as hemodynamic properties of the aorta are associated with cognitive function in post-menopausal females, but a history of preeclampsia influences this association for some cognitive abilities (70). Therefore, more research is needed to understand how sex may influence the effects of large artery stiffness on cognitive function.
9. Perspectives: a two-hit hypothesis for female brain aging and remaining gaps in knowledge
The current hypothesis is that an age-related increase in large artery stiffness and pulse pressure leads to cerebrovascular and cognitive impairment. As the age-related stiffening of the large arteries is slower to progress in males, this may allow time for adaptation of the cerebral vasculature to elevated pulse pressure. In females, post-menopause, there is a more rapid increase in arterial stiffness, and this coincides with the loss of estrogen’s protective effects on endothelial cells. Thus, early post-menopausal females are susceptible to two-hits simultaneously that can lead to cerebrovascular and cognitive impairment, and this may explain the increased AD risk in females.
A few factors have led to the paucity of data regarding sex differences in the effects of large artery stiffness on AD-related outcomes, such as the historical exclusion of females from studies and the treatment of sex as a confounding variable rather than an important contributor to physiology. In addition, ovariectomy is often used to induce a menopause-like state in young rodents matching human surgically induced menopause; however, this is distinctly different from natural human menopause as 1) the effects of estrogen deficiency may impact young and old females differently, and 2) human menopause typically does not have a sudden onset of estrogen loss (71). Lastly, differences in the age of subjects may contribute to inconsistencies, as the rapid increase in arterial stiffness and cognitive decline are typically only found before the age of 75 years in females. Therefore, future studies need to include females in peri- and early post-menopause to understand these key physiological changes.
10. Conclusions
Age-related increases in large artery stiffness are associated with cerebral endothelial cell dysfunction, reduced cerebral blood flow, neuropathology, and cognitive impairment. As females experience a more rapid increase in large artery stiffness with aging, coinciding with menopause, they could be more susceptible to these damaging effects, and this may explain their increased risk for AD (Figure 1). These deleterious effects of increased large artery stiffness in older females likely contribute to other neurological diseases in addition to AD. Numerous efforts, in both human and animal studies, are needed to close the gaps in knowledge about the effects of vascular aging on the female brain.
Figure 1:
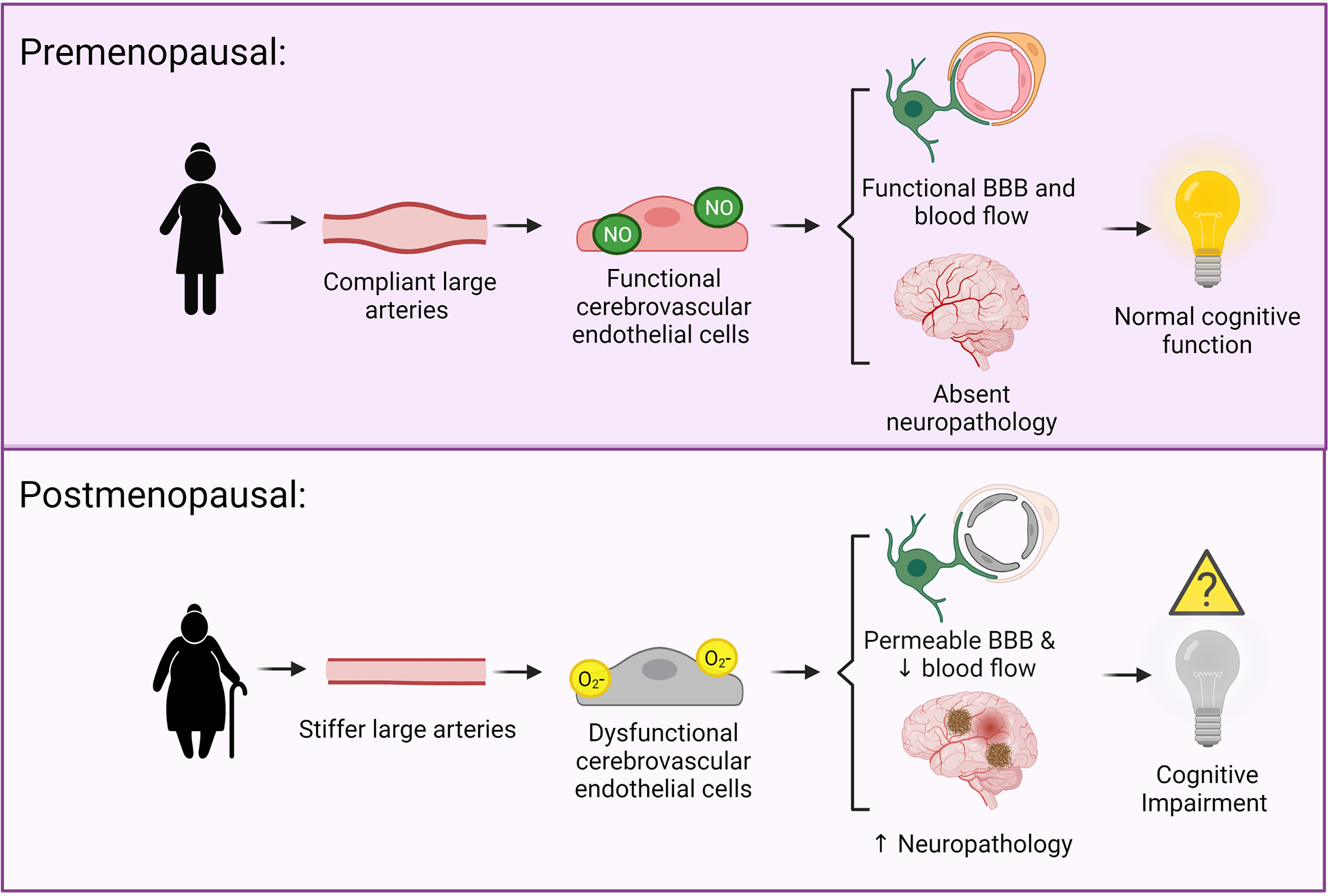
Hypothesized mechanisms linking large artery stiffness and cognitive impairment. Above: In premenopausal females, the large arteries are compliant and cerebral pressure and blood flow pulsatility is low. This is associated with functional cerebral endothelial cells, a functional blood brain barrier, adequate cerebral blood flow, and an absence of neuropathology. Below: In postmenopausal females, there is greater large artery stiffness and higher cerebral pressure and blood flow pulsatility. This is associated with dysfunction of the cerebral endothelial cells, a more permeable blood brain barrier, neurovascular uncoupling, reduced cerebral blood flow, and increased neuropathology. Figure created with Biorender.com
12. Funding
This work was supported by National Institutes of Health R01 AG064016 and the John L Luvaas Family Fund.
13 References
- 1.Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9(2):208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Lin KA, Choudhury KR, Rathakrishnan BG, et al. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2015;1(2):103–110. doi: 10.1016/j.trci.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapasi A, Schneider JA. Vascular contributions to cognitive impairment, clinical Alzheimer’s disease, and dementia in older persons. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2016;1862(5):878–886. doi: 10.1016/j.bbadis.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iulita MF, Noriega de la Colina A, Girouard H. Arterial stiffness, cognitive impairment and dementia: confounding factor or real risk? J Neurochem. 2018;144(5):527–548. doi: 10.1111/jnc.14235. [DOI] [PubMed] [Google Scholar]
- 5.Coutinho T Arterial stiffness and its clinical implications in women. Can J Cardiol. 2014;30(7):756–764. doi: 10.1016/j.cjca.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol. 2019;116:135–170. doi: 10.1016/bs.apcsb.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmerman MA, Budish RA, Kashyap S, Lindsey SH. GPER - novel membrane estrogen receptor. Clin Sci (Lond). 2016;130(12):1005–1016. doi: 10.1042/CS20160114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morissette M, Le Saux M, D’Astous M, et al. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. The Journal of Steroid Biochemistry and Molecular Biology. 2008;108(3):327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Pau CY, Pau K-YF, Spies HG. Putative estrogen receptor β and α mRNA expression in male and female rhesus macaques. Molecular and Cellular Endocrinology. 1998;146(1):59–68. doi: 10.1016/S0303-7207(98)00197-X. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ, Lenton EA, Sexton L, Cooke ID. The effect of age on the cyclical patterns of plasma LH, FSH, oestradiol and progesterone in women with regular menstrual cycles. Human Reproduction. 1988;3(7):851–855. doi: 10.1093/oxfordjournals.humrep.a136796. [DOI] [PubMed] [Google Scholar]
- 11.Morley JE. Androgens and aging. Maturitas. 2001;38(1):61–71. doi: 10.1016/S0378-5122(00)00192-4. [DOI] [PubMed] [Google Scholar]
- 12.Berry KL, Cameron JD, Dart AM, et al. Large-Artery Stiffness Contributes to the Greater Prevalence of Systolic Hypertension in Elderly Women. Journal of the American Geriatrics Society. 2004;52(3):368–373. doi: 10.1111/j.1532-5415.2004.52107.x. [DOI] [PubMed] [Google Scholar]
- 13.Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA. Women exhibit a greater age-related increase in proximal aortic stiffness than men. J Hypertens. 2001;19(12):2205–2212. doi: 10.1097/00004872-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y, Pechlaner R, Cai J, et al. Trajectories of Age-Related Arterial Stiffness in Chinese Men and Women. J Am Coll Cardiol. 2020;75(8):870–880. doi: 10.1016/j.jacc.2019.12.039. [DOI] [PubMed] [Google Scholar]
- 15.DuPont JJ, Kim SK, Kenney RM, Jaffe IZ. Sex differences in the time course and mechanisms of vascular and cardiac aging in mice: role of the smooth muscle cell mineralocorticoid receptor. American Journal of Physiology-Heart and Circulatory Physiology. 2021;320(1):H169–H180. doi: 10.1152/ajpheart.00262.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scuteri A, Lakatta EG, Bos AJG, Fleg JL. Effect of estrogen and progestin replacement on arterial stiffness indices in postmenopausal women. Aging Clin Exp Res. 2001;13(2):122–130. doi: 10.1007/BF03351534. [DOI] [PubMed] [Google Scholar]
- 17.Fonck E, Feigl GG, Fasel J, et al. Effect of Aging on Elastin Functionality in Human Cerebral Arteries. Stroke. 2009;40(7):2552–2556. doi: 10.1161/STROKEAHA.108.528091. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi K, Hirayama E. Age-related changes of wall composition and collagen cross-linking in the rat carotid artery – In relation with arterial mechanics. Journal of the Mechanical Behavior of Biomedical Materials. 2017;65:881–889. doi: 10.1016/j.jmbbm.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Qiu H, Depre C, Ghosh K, et al. Mechanism of Gender-Specific Differences in Aortic Stiffness With Aging in Nonhuman Primates. Circulation. 2007;116(6):669–676. doi: 10.1161/CIRCULATIONAHA.107.689208. [DOI] [PubMed] [Google Scholar]
- 20.Natoli AK, Medley TL, Ahimastos AA, et al. Sex Steroids Modulate Human Aortic Smooth Muscle Cell Matrix Protein Deposition and Matrix Metalloproteinase Expression. Hypertension. 2005;46(5):1129–1134. doi: 10.1161/01.HYP.0000187016.06549.96. [DOI] [PubMed] [Google Scholar]
- 21.Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic Acid Selectively Improves Large Elastic Artery Compliance in Postmenopausal Women. Hypertension. 2005;45(6):1107–1112. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- 22.Moreau KL, Meditz A, Deane KD, Kohrt WM. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. American Journal of Physiology-Heart and Circulatory Physiology. 2012;302(5):H1211–H1218. doi: 10.1152/ajpheart.01065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey RE, Barnes JN, Hart ECJ, Nicholson WT, Joyner MJ, Casey DP. Influence of sympathetic nerve activity on aortic hemodynamics and pulse wave velocity in women. Am J Physiol Heart Circ Physiol. 2017;312(2):H340–H346. doi: 10.1152/ajpheart.00447.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holwerda SW, Luehrs RE, DuBose L, et al. Elevated Muscle Sympathetic Nerve Activity Contributes to Central Artery Stiffness in Young and Middle-Age/Older Adults. Hypertension. 2019;73(5):1025–1035. doi: 10.1161/HYPERTENSIONAHA.118.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreau KL, Hildreth KL. Vascular Aging across the Menopause Transition in Healthy Women. Adv Vasc Med. 2014;2014:204390. doi: 10.1155/2014/204390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DuPont JJ, Kenney RM, Patel AR, Jaffe IZ. Sex differences in mechanisms of arterial stiffness. British Journal of Pharmacology. 2019;176(21):4208–4225. doi: 10.1111/bph.14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell GF. Aortic stiffness, pressure and flow pulsatility, and target organ damage. Journal of Applied Physiology. 2018;125(6):1871–1880. doi: 10.1152/japplphysiol.00108.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Montgolfier O, Pinçon A, Pouliot P, et al. High Systolic Blood Pressure Induces Cerebral Microvascular Endothelial Dysfunction, Neurovascular Unit Damage, and Cognitive Decline in Mice. Hypertension. 2019;73(1):217–228. doi: 10.1161/HYPERTENSIONAHA.118.12048. [DOI] [PubMed] [Google Scholar]
- 29.Alwatban MR, Aaron SE, Kaufman CS, et al. Effects of age and sex on middle cerebral artery blood velocity and flow pulsatility index across the adult lifespan. Journal of Applied Physiology. 2021;130(6):1675–1683. doi: 10.1152/japplphysiol.00926.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lefferts WK, DeBlois JP, Augustine JA, Keller AP, Heffernan KS. Age, sex, and the vascular contributors to cerebral pulsatility and pulsatile damping. Journal of Applied Physiology. 2020;129(5):1092–1101. doi: 10.1152/japplphysiol.00500.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daneman R, Prat A. The Blood–Brain Barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanhoutte PM, Shimokawa H, Feletou M, Tang EHC. Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiol. 2017;219(1):22–96. doi: 10.1111/apha.12646. [DOI] [PubMed] [Google Scholar]
- 33.Donato AJ, Morgan RG, Walker AE, Lesniewski LA. Cellular and Molecular Biology of Aging Endothelial Cells. J Mol Cell Cardiol. 2015;89(0 0):122–135. doi: 10.1016/j.yjmcc.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21(10):1318–1331. doi: 10.1038/s41593-018-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330(6012):1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. Journal of Applied Physiology. 2006;101(4):1252–1261. doi: 10.1152/japplphysiol.01095.2005. [DOI] [PubMed] [Google Scholar]
- 37.Haynes MP, Sinha D, Russell KS, et al. Membrane Estrogen Receptor Engagement Activates Endothelial Nitric Oxide Synthase via the PI3-Kinase–Akt Pathway in Human Endothelial Cells. Circulation Research. 2000;87(8):677–682. doi: 10.1161/01.RES.87.8.677. [DOI] [PubMed] [Google Scholar]
- 38.Torres MJ, Kew KA, Ryan TE, et al. 17β-Estradiol Directly Lowers Mitochondrial Membrane Microviscosity and Improves Bioenergetic Function in Skeletal Muscle. Cell Metabolism. 2018;27(1):167–179.e7. doi: 10.1016/j.cmet.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strehlow K, Rotter S, Wassmann S, et al. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res. 2003;93(2):170–177. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]
- 40.Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular Endothelial Estrogen Receptor α Is Modulated by Estrogen Status and Related to Endothelial Function and Endothelial Nitric Oxide Synthase in Healthy Women. The Journal of Clinical Endocrinology & Metabolism. 2009;94(9):3513–3520. doi: 10.1210/jc.2009-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taddei S, Virdis A, Ghiadoni L, et al. Menopause Is Associated With Endothelial Dysfunction in Women. Hypertension. 1996;28(4):576–582. doi: 10.1161/01.HYP.28.4.576. [DOI] [PubMed] [Google Scholar]
- 42.Robison LS, Gannon OJ, Salinero AE, Zuloaga KL. Contributions of sex to cerebrovascular function and pathology. Brain Res. 2019;1710:43–60. doi: 10.1016/j.brainres.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 43.Delli Gatti C, Osto E, Kouroedov A, et al. Pulsatile stretch induces release of angiotensin II and oxidative stress in human endothelial cells: effects of ACE inhibition and AT1 receptor antagonism. Clin Exp Hypertens. 2008;30(7):616–627. doi: 10.1080/10641960802443183. [DOI] [PubMed] [Google Scholar]
- 44.Raignault A, Bolduc V, Lesage F, Thorin E. Pulse pressure-dependent cerebrovascular eNOS regulation in mice. J Cereb Blood Flow Metab. 2017;37(2):413–424. doi: 10.1177/0271678X16629155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Girão-Silva T, Fonseca-Alaniz MH, Ribeiro-Silva JC, et al. High stretch induces endothelial dysfunction accompanied by oxidative stress and actin remodeling in human saphenous vein endothelial cells. Sci Rep. 2021;11:13493. doi: 10.1038/s41598-021-93081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker AE, Henson GD, Reihl KD, et al. Greater impairments in cerebral artery compared with skeletal muscle feed artery endothelial function in a mouse model of increased large artery stiffness. J Physiol. 2015;593(Pt 8):1931–1943. doi: 10.1113/jphysiol.2014.285338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muhire G, Iulita MF, Vallerand D, et al. Arterial Stiffness Due to Carotid Calcification Disrupts Cerebral Blood Flow Regulation and Leads to Cognitive Deficits. Journal of the American Heart Association. 2019;8(9):e011630. doi: 10.1161/JAHA.118.011630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu CK, Lyass A, Larson MG, et al. Biomarkers of oxidative stress are associated with frailty: the Framingham Offspring Study. Age (Dordr). 2016;38(1). doi: 10.1007/s11357-015-9864-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aanerud J, Borghammer P, Rodell A, Jónsdottir KY, Gjedde A. Sex differences of human cortical blood flow and energy metabolism. J Cereb Blood Flow Metab. 2017;37(7):2433–2440. doi: 10.1177/0271678X16668536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DuBose LE, Boles Ponto LL, Moser DJ, Harlynn E, Reierson L, Pierce GL. Higher Aortic Stiffness Is Associated With Lower Global Cerebrovascular Reserve Among Older Humans. Hypertension. 2018;72(2):476–482. doi: 10.1161/HYPERTENSIONAHA.118.11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kastrup A, Dichgans J, Niemeier M, Schabet M. Changes of Cerebrovascular CO 2 Reactivity During Normal Aging. Stroke. 1998;29(7):1311–1314. doi: 10.1161/01.STR.29.7.1311. [DOI] [PubMed] [Google Scholar]
- 52.Barnes JN, Charkoudian N. Integrative cardiovascular control in women: Regulation of blood pressure, body temperature, and cerebrovascular responsiveness. The FASEB Journal. 2021;35(2):e21143. doi: 10.1096/fj.202001387R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jefferson AL, Cambronero FE, Liu D, et al. Higher Aortic Stiffness Is Related to Lower Cerebral Blood Flow and Preserved Cerebrovascular Reactivity in Older Adults. Circulation. 2018;138(18):1951–1962. doi: 10.1161/CIRCULATIONAHA.118.032410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knutsen RH, Beeman SC, Broekelmann TJ, et al. Minoxidil improves vascular compliance, restores cerebral blood flow, and alters extracellular matrix gene expression in a model of chronic vascular stiffness. American Journal of Physiology-Heart and Circulatory Physiology. 2018;315(1):H18–H32. doi: 10.1152/ajpheart.00683.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hughes TM, Wagenknecht LE, Craft S, et al. Arterial stiffness and dementia pathology: Atherosclerosis Risk in Communities (ARIC)-PET Study. Neurology. 2018;90(14):e1248–e1256. doi: 10.1212/WNL.0000000000005259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poels MMF, Ikram MA, Lugt A van der, et al. Cerebral microbleeds are associated with worse cognitive function: The Rotterdam Scan Study. Neurology. 2012;78(5):326–333. doi: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- 57.Rensma SP, van Sloten TT, Houben AJHM, et al. Microvascular Dysfunction Is Associated With Worse Cognitive Performance. Hypertension. 2020;75(1):237–245. doi: 10.1161/HYPERTENSIONAHA.119.13023. [DOI] [PubMed] [Google Scholar]
- 58.Rosano C, Watson N, Chang Y, et al. Aortic Pulse Wave Velocity Predicts Focal White Matter Hyperintensities in a Biracial Cohort of Older Adults. Hypertension. 2013;61(1):160–165. doi: 10.1161/HYPERTENSIONAHA.112.198069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitchell GF, van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility – Reykjavik Study. Brain. 2011;134(11):3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnes JN, Harvey RE, Zuk SM, et al. Aortic hemodynamics and white matter hyperintensities in normotensive postmenopausal women. J Neurol. 2017;264(5):938–945. doi: 10.1007/s00415-017-8476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sadekova N, Iulita MF, Vallerand D, et al. Arterial stiffness induced by carotid calcification leads to cerebral gliosis mediated by oxidative stress. J Hypertens. 2018;36(2):286–298. doi: 10.1097/HJH.0000000000001557. [DOI] [PubMed] [Google Scholar]
- 62.Reas ET, Laughlin GA, Hagler DJ, Lee RR, Dale AM, McEvoy LK. Age and Sex Differences in the Associations of Pulse Pressure With White Matter and Subcortical Microstructure. Hypertension. 2021;77(3):938–947. doi: 10.1161/HYPERTENSIONAHA.120.16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiss EM, Kemmler G, Deisenhammer EA, Fleischhacker WW, Delazer M. Sex differences in cognitive functions. Personality and Individual Differences. 2003;35(4):863–875. doi: 10.1016/S0191-8869(02)00288-X. [DOI] [Google Scholar]
- 64.Hanon O, Haulon S, Lenoir H, et al. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke. 2005;36(10):2193–2197. doi: 10.1161/01.STR.0000181771.82518.1c. [DOI] [PubMed] [Google Scholar]
- 65.Tarumi T, Shah F, Tanaka H, Haley AP. Association between central elastic artery stiffness and cerebral perfusion in deep subcortical gray and white matter. Am J Hypertens. 2011;24(10):1108–1113. doi: 10.1038/ajh.2011.101. [DOI] [PubMed] [Google Scholar]
- 66.Pase MP, Beiser A, Himali JJ, et al. Aortic Stiffness and the Risk of Incident Mild Cognitive Impairment and Dementia. Stroke. 2016;47(9):2256–2261. doi: 10.1161/STROKEAHA.116.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meyer ML, Palta P, Tanaka H, et al. Association of Central Arterial Stiffness and Pressure Pulsatility with Mild Cognitive Impairment and Dementia: The Atherosclerosis Risk in Communities Study-Neurocognitive Study (ARIC-NCS). J Alzheimers Dis. 2017;57(1):195–204. doi: 10.3233/JAD-161041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rouch L, Cestac P, Sallerin B, et al. Pulse Wave Velocity Is Associated With Greater Risk of Dementia in Mild Cognitive Impairment Patients. Hypertension. 2018;72(5):1109–1116. doi: 10.1161/HYPERTENSIONAHA.118.11443. [DOI] [PubMed] [Google Scholar]
- 69.Singer J, Trollor JN, Crawford J, et al. The Association between Pulse Wave Velocity and Cognitive Function: The Sydney Memory and Ageing Study. PLOS ONE. 2013;8(4):e61855. doi: 10.1371/journal.pone.0061855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller VM, Jayachandran M, Barnes JN, Mielke MM, Kantarci K, Rocca WA. Risk factors of neurovascular ageing in women. Journal of Neuroendocrinology. 2020;32(1):e12777. doi: 10.1111/jne.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diaz Brinton R Minireview: Translational Animal Models of Human Menopause: Challenges and Emerging Opportunities. Endocrinology. 2012;153(8):3571–3578. doi: 10.1210/en.2012-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]


