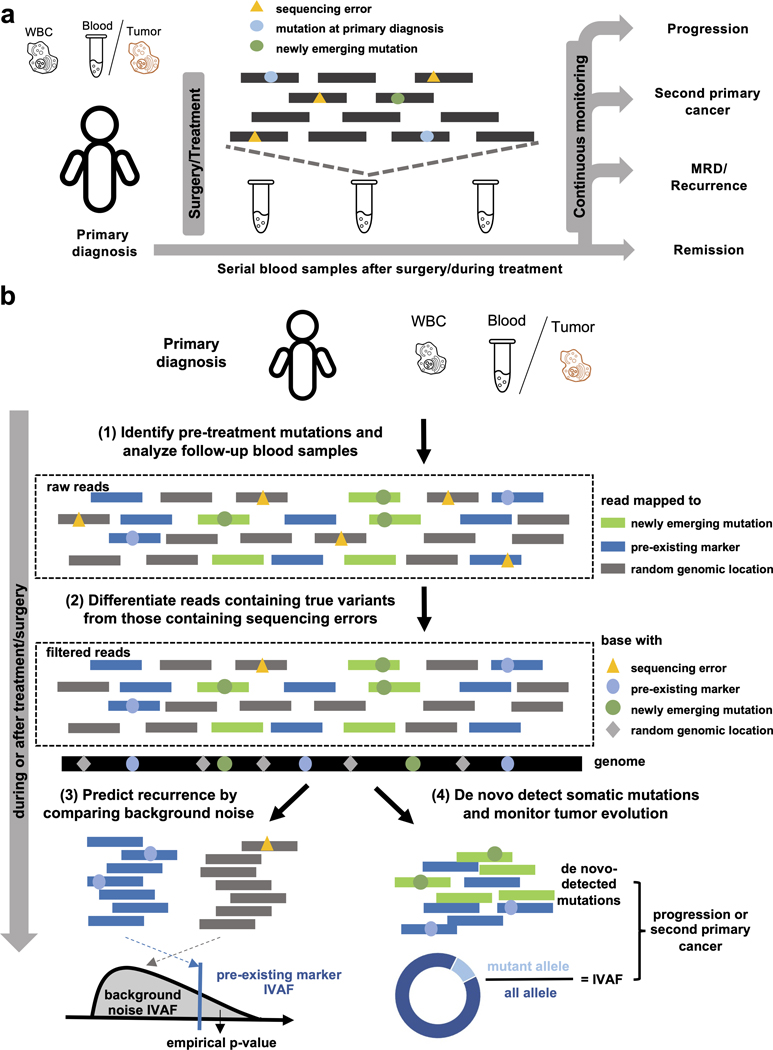
Figure 1. Cancer monitoring in plasma samples by tracking pre-existing tumor mutations and newly emerging tumor mutations.
(a) Illustration of the sample collection for cfDNA-based cancer monitoring. Prior to surgery or therapy, a plasma or tumor sample and a white blood cell (WBC) sample are collected to generate the pre-existing tumor profile. Serial blood samples are collected to detect MRD/recurrence and monitor tumor evolution after treatment. (b) Illustration of the method workflow. In the pre-treatment samples, clonal tumor mutations are identified for tumor tracking in the post-treatment samples. Given a post-treatment plasma sample, the tumor fraction is calculated from the pre-existing clonal tumor mutations and compared to a sample-specific background distribution. The empirical p-value of the tumor fraction is used to predict MRD/recurrence. Furthermore, de novo somatic mutations are detected using cfSNV between the post-treatment plasma and WBC samples. A second primary cancer is predicted by a logistic regression model that accounts for both the amount of de novo mutations and the corresponding tumor fraction.