FIGURE 3.
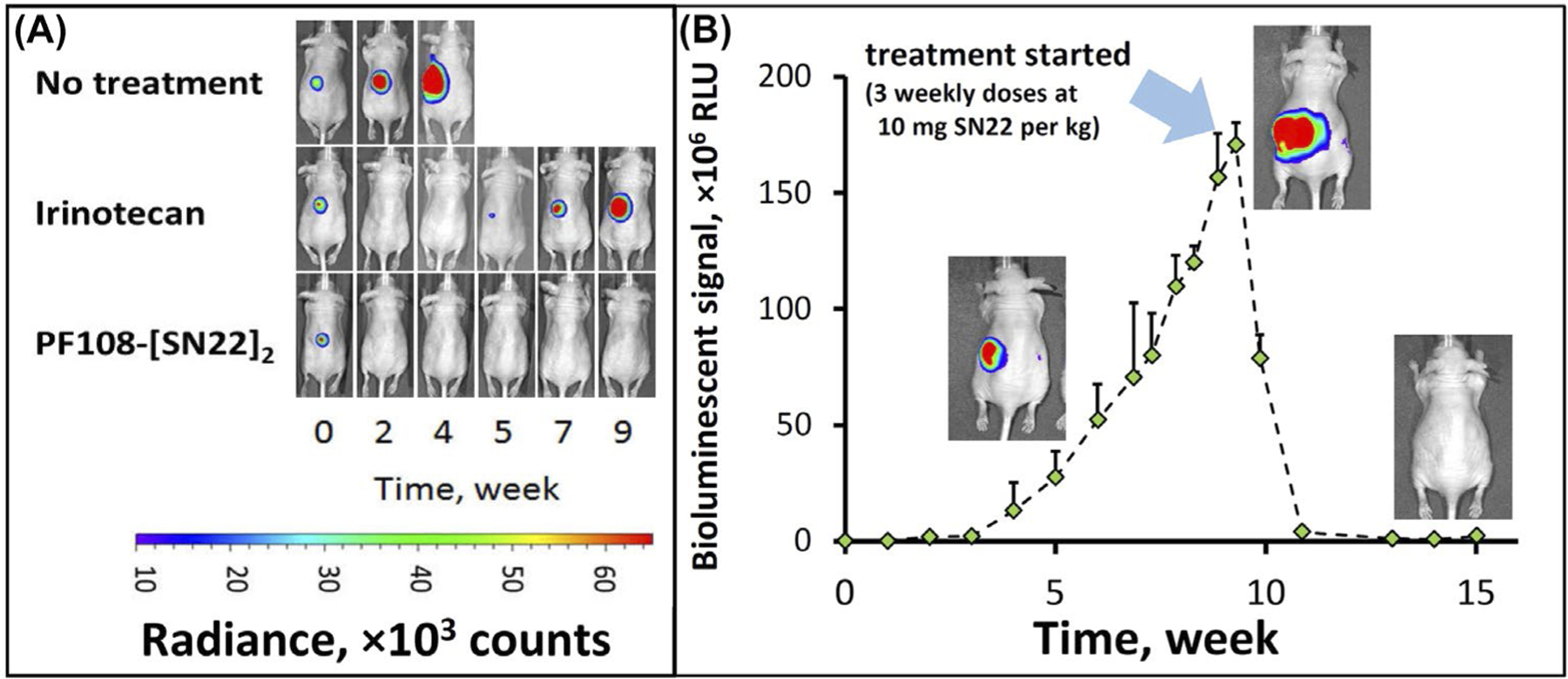
Therapeutic efficacy of PF108-[SN22]2 in an orthotopic mouse model of newly diagnosed, MYCN-driven NB. Athymic nude mice (nu/nu) were inoculated with 106 luciferase-expressing IMR-32 cells in the perirenal fat pad. Treatment with irinotecan or PF108-[SN22]2 at doses corresponding to 10 mg of SN38 or SN22 per kg (2× and 1× week, respectively, over 4 weeks) was initiated 3 weeks after inoculation (A). Tumor-associated signal was monitored by quantitative bioluminescence. Although animals in the irinotecan group initially responded by tumor regression, the response did not extend beyond the treatment period. In contrast, PF108-[SN22]4 caused complete tumor disappearance in 4 and a durable partial response in 1 out of 5 animals (the detailed tumor monitoring analysis and animal survival are shown in Figure S4 in Supporting Information). In an additional experiment, PF108-[SN22]2 administered 1× week over 3 weeks to animals approaching the endpoint with 10-fold larger NB tumors achieved durable responses and extended event-free survival (B). Data are shown as mean ± SD
