Figure 2. Mechanisms of direct and indirect microbial sensing by HSPCs.
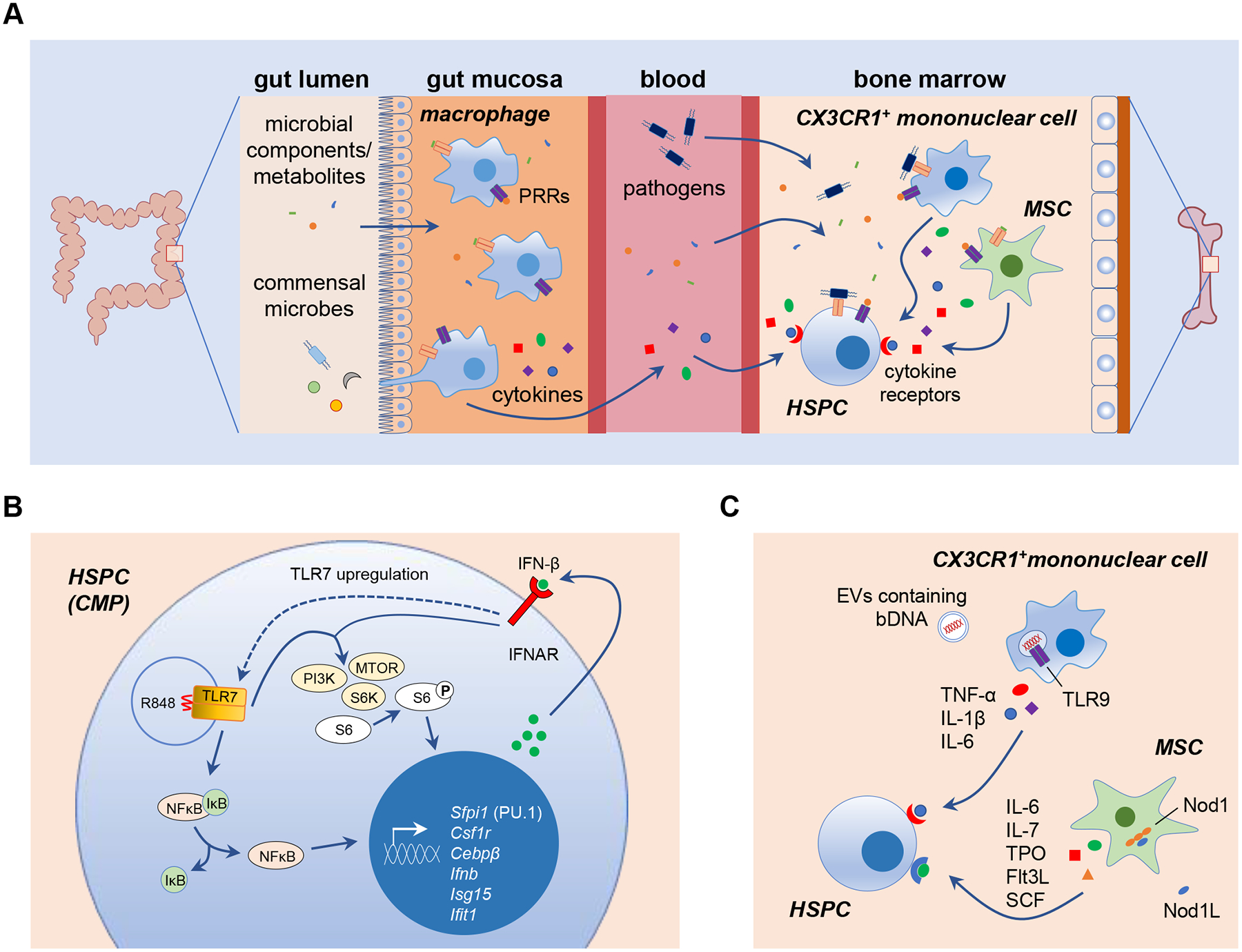
(A) Direct and indirect mechanisms of microbial sensing regulate HSPC maintenance and differentiation. Microbial components and metabolites derived from the microbiome are present in the circulation, so it is possible that they, as well as invading pathogens and their components, can be sensed directly by bone marrow HSPCs via their PRRs, including TLRs. HSC niche cells, such as mesenchymal stromal cells (MSCs) and CX3CR1+ mononuclear cells, can also detect microbes using PRRs and release pro-inflammatory cytokines to regulate HSPCs. Circulating cytokines released by mucosal macrophages sensing gut microbiome-derived components and metabolites may also be detected by HSPCs in the bone marrow. DAMPs produced by dying cells or damaged tissues may also be detected by HSPC PRRs. (B) During emergency myelopoiesis induced by the TLR7 agonist R848, direct detection by common myeloid progenitors (CMPs) stimulates NFκB-mediated induction of myeloid lineage genes such as Sfpi1 (PU.1), Csf1r and Cebpβ. NFκB also induces IFN-β production, and autocrine detection of IFN-β by IFNAR induces TLR7 upregulation and synergistic activation of PI3K-mTOR signaling to promote CMP differentiation into macrophages [46]. (C) One mechanism proposed for microbiome-mediated HSPC regulation in the steady-state is detection of commensal bacterial DNA (bDNA) by bone marrow CX3CR1+ mononuclear cells (presumably via endocytic TLR9) following delivery of the bDNA from the gut in extracellular vesicles (EVs) [58]. Microbiome-derived Nod1 ligands (Nod1L) are also sensed by MSCs [13]. Cytokines produced by these cells promote HSPC proliferation and differentiation to maintain steady-state hematopoiesis.
