Abstract
Background:
COVID-19 has affected every country globally, with hundreds of millions of people infected with the SARS-CoV-2 virus and over 6 million deaths to date. It is unknown how alcohol use disorder (AUD) may affect the severity and mortality of COVID-19. AUDs are known to increase the severity and mortality of bacterial pneumonia and the risk of developing acute respiratory distress syndrome (ARDS). Our objective is to determine whether those with AUDs have increased severity and mortality from COVID-19.
Methods:
We utilized a retrospective cohort study of inpatients and outpatients from 44 centers participating in the National COVID Cohort Collaborative (N3C). All were adult COVID-19 patients with and without documented AUDs
Results:
We identified 25,583 COVID-19 patients with AUDs and 1,309,445 without. In unadjusted comparisons, those with AUD had higher odds of hospitalization (odds ratio [OR] 2.00, 95% confidence interval [CI]1.94–2.06, p<0.001). After adjusting for age, sex, race/ethnicity, smoking, BMI, and comorbidities, those with an AUD still had higher odds of requiring hospitalization (adjusted OR [aOR] 1.51, CI 1.46–1.56, p<0.001). In unadjusted comparisons, those with AUD had higher odds of all-cause mortality (OR 2.18, CI 2.05–2.31, p<0.001) After adjusting as above, those with an AUD still had higher odds of all-cause mortality (aOR 1.55, CI 1.46–1.65, p<0.001).
Conclusion:
This work suggests that AUDs can increase the severity and mortality of COVID-19 infection. This reinforces that clinicians should obtain an accurate alcohol history from patients admitted with COVID-19. For this study, our results are limited by our inability to quantify the daily drinking habits of the participants. Further studies are needed to determine the mechanisms of how AUDs increase the severity and mortality of COVID-19.
Keywords: Alcoholic, Ethanol, SARS-CoV-2, viral pneumonia, ARDS, smoking
INTRODUCTION
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has swept the globe, with over 173 million cases and over 6 million deaths worldwide to date (Dong et al., 2020). Several factors have been shown to increase mortality in COVID-19 pneumonia. These include advanced age (Mueller et al., 2020), male sex (Peckham et al., 2020), obesity (Popkin et al., 2020), smoking (van Zyl-Smit et al., 2020), pre-existing lung disease (Esposito et al., 2020, Pranata et al., 2020b), and the number of comorbidities (Knight et al., 2020).
Several groups suggest that heavy alcohol intake might predispose COVID-19 patients to poor clinical outcomes (Bailey et al., 2021, Testino, 2020, Saengow et al., 2021). This suggestion, however, has remained only conjecture because alcohol intake was not assessed in most studies of risk factors for COVID-19 infection and severity (Jordan et al., 2020, Gao et al., 2021, Chang et al., 2020, Zheng et al., 2020, Li et al., 2020). This omission is despite the fact that over 2.4 billion people (43% of the total population) worldwide consume alcohol regularly (Organization, 2019).
Heavy alcohol intake and alcohol use disorder (AUD) are also known to have profound effects on the innate and adaptive immunity of the lung (Bailey et al., 2019). In terms of innate immunity, alcohol can suppress cough (Calesnick and Vernick, 1971) and blunt mucociliary clearance from the lung (Sisson et al., 2005). Cytokine production, another component of the innate immune response, is altered in those with AUD. For instance, the chemokine RANTES (Burnham et al., 2013) and the inflammatory cytokines IL-6 and IL-8 (Bailey et al., 2015) were increased in the bronchoalveolar lavage (BAL) fluid of those with AUD. These changes in the lung alter the earliest responses to infection, which can potentially change the lung’s defense against viral infection. In animal models of pulmonary viral infections, alcohol administration is associated with changes that make infection more likely (Simet et al., 2013). In addition, alcohol administration is associated with increased morbidity and mortality in the animals (Meyerholz et al., 2008) as well as higher viral titers and more severe pathology (Jerrells et al., 2007, Warren et al., 2016, Warren et al., 2019).
In humans, AUDs are known to be an independent risk factor for the development of community-acquired bacterial pneumonia. This is thought to be dose-dependent, and it is estimated that for every 10–20g increase in daily alcohol consumption, there is an 8% increase in the risk of developing community-acquired pneumonia (Simou et al., 2018a). This corresponds to an increase of approximately one standard drink (14 grams of alcohol) which is contained in one 12 oz beer, a 1.5 oz shot of liquor or 5 oz of wine.
Those with AUD are also more likely to develop acute respiratory distress syndrome (ARDS) (TenHoor et al., 2001, Moss et al., 2003, Moss and Burnham, 2003, Moss and Burnham, 2006). ARDS is a condition of respiratory failure due to the rapid development of non-cardiogenic pulmonary edema. ARDS is diagnosed frequently in cases of severe COVID-19 pneumonia that frequently requires mechanical ventilation (Marini and Gattinoni, 2020). Patients with ARDS who drink more than three alcoholic drinks per week have higher mortality (TenHoor et al., 2001).
It is not known how AUD impacts the severity of COVID-19 disease. Given what is known about alcohol increasing the severity of other lung infections and ARDS, we hypothesized that AUD would increase hospitalization and mortality in COVID-19.
METHODS
National COVID Cohort Collaborative (N3C Initiative)
The National COVID Cohort Collaborative (N3C) is a centralized, secure repository of clinical data housed at, and managed by, the National Institutes of Health (NIH) National Center for Advancing Translational Science (NCATS). N3C aggregates and harmonizes electronic health record (EHR) data across clinical organizations in the United States and is designed to support collaborative, community-driven, reproducible, and transparent COVID-19 secondary data analyses. As of October 1, 2021, N3C is the largest central repository of COVID-19 electronic health records in the United States with records for more than 8 million patients, of which, 2.8 million tested positive for COVID-19 from 65 institutions (i.e. data partners). These institutions are listed in Supplementary Table 1.
N3C data enclave
The N3C data enclave is a secure platform through which harmonized clinical data is provided by contributing data partners. The N3C systematically collects data derived from the electronic medical record of patients that were tested for SARS-CoV-2 or had a diagnosis of COVID-19 from a provider.
The data enclave is described in detail here (Haendel et al., 2021). Briefly, the data enclave includes Electronic Health Record (EHR) or Health Information Exchange (HIE) data (with a 2-year “lookback” to January 1, 2018) on all patients with COVID-19. As of September 30, 2021 (release 47), this includes data from 65 data partners representing 8,350,600 patients, of whom 2,856,925 were diagnosed with COVID-19. Data are collected as part of routine medical care and submitted to the N3C database under a Health Insurance and Portability and Accountability Act (HIPAA) waiver via one of 4 common data models (CDMs): Observational Medical Outcomes Partnership (OMOP) (Dixon et al., 2020), Patient-Centered Clinical Research Network (PCORnet) (Bian et al., 2020), TriNetX (Topaloglu and Palchuk, 2018), or Accrual to Clinical Trials (ACT) (Visweswaran et al., 2018). Submitted data are then checked for quality using methods tailored to each of the 4 CDMs before being harmonized into the OMOP 5.3.1.8 CDM followed by additional data quality checks prior to release (Haendel et al., 2021). Harmonization includes mapping codes, such as ICD-10 or SNOMED, to common concepts defined in the OMOP CDM. Submitted data is ingested and released on a weekly basis.
Research Ethics
Data collection activities for N3C are approved under the authority of the NIH Institutional Review Board (IRB, IRB00249128) with Johns Hopkins University School of Medicine as the central IRB. Institutional IRBs at each data partner either approved the study protocol or ceded to this single IRB. The University of Nebraska Medical Center contributed data and completed a data use agreement (DUA) with the N3C. The investigators for this study also completed a data use request (DUR) that was approved by the N3C (RP-E5D34E). We used a de-identified dataset that did not contain direct identifiers of individual patients such as name, medical record number, date of birth, date(s) of service (all dates are shifted by up to 180 days), site, address or zip codes, or any other HIPAA identifier. The investigators agreed to abide by the N3C data user code of conduct. This code of conduct included an agreement not to attempt to re-identify any individual or site. Additional information can be found in Supplementary Methods.
Inclusion and exclusion criteria for data partners
Data partners reporting data to the N3C have varying levels of data completeness. To overcome this problem, we developed a data robustness screening matrix to determine minimum fact reporting per patient across key domains. This follows a similar approach used by the four source data models, which all rely on data quality dashboards to enhance site reporting for inclusion into network studies: OMOP, ACT, TriNetX, and PCORnet.
We excluded data partners that did not provide sufficient data to calculate BMI for at least 33 percent of their patients and those that reported limited death information, resulting in 44 out of 65 possible data partners being included in our final analyses. Once we finalized the set of reliable data partners, we included patients diagnose with COVID-19 or a positive lab test for SARS-CoV-2 who were 19 years old or older. Patients with missing age or gender were excluded. Figure 2 includes a flow chart of study inclusion and exclusion criteria.
Figure 2:
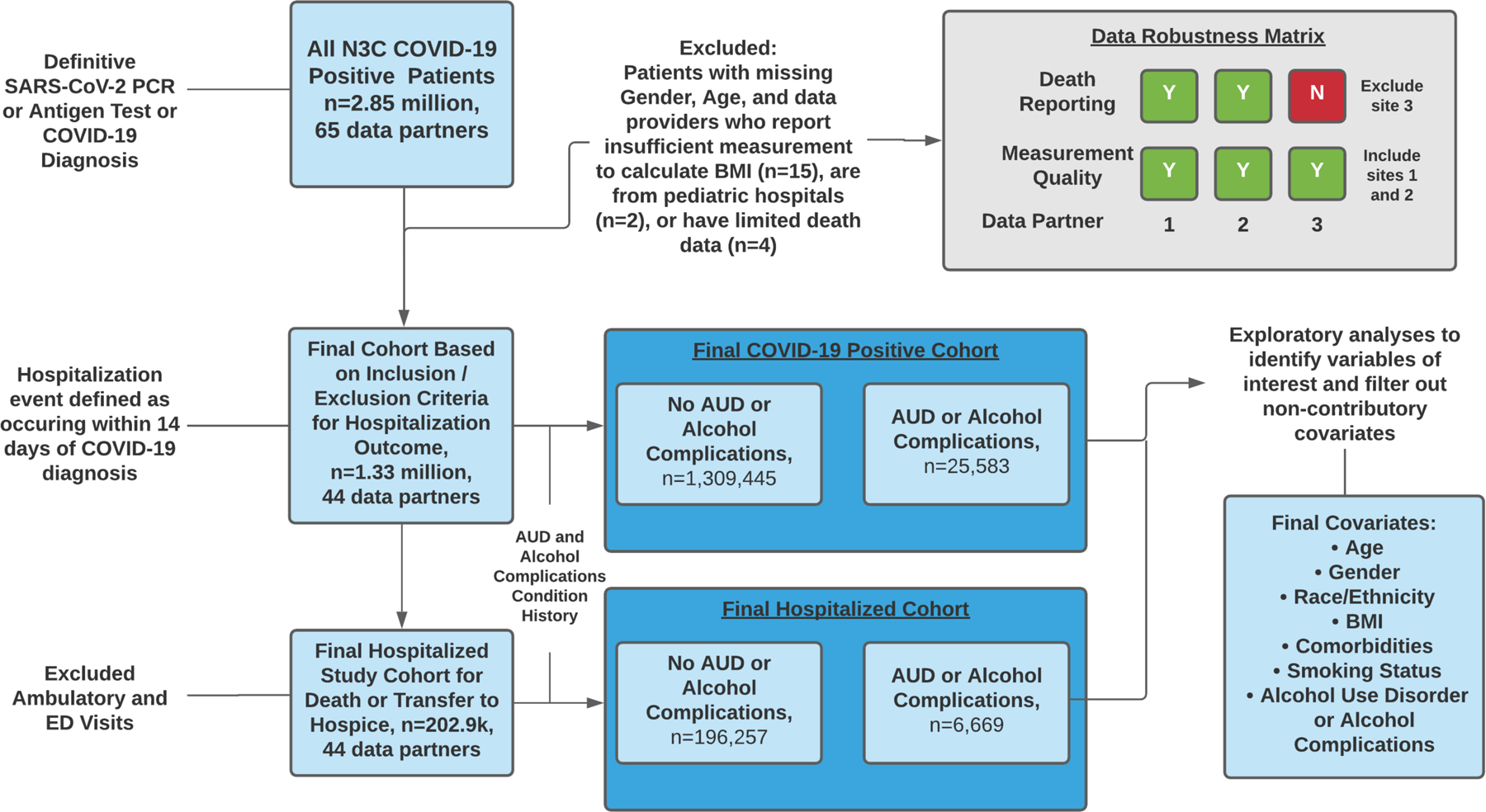
Summary of the analysis strategy. Abbreviations used: ED emergency department, AUD alcohol use disorder, BMI body mass index.
Data Extraction from the N3C Enclave
Data was extracted on October 1, 2021, (N3C release 47) in the OMOP Common Data Model version 5.3.1. All clinical concept sets were created collaboratively within the N3C Enclave, with at least one informatician and one clinical subject-matter expert reviewing each relevant concept set. Concept sets contain standardized terminology corresponding to clinical domains (e.g., LOINC, SNOMED CT, ICD-10, RxNorm). For more information on the N3C enclave, data characteristics, data analysis and cohort definition in the N3C data enclave, please refer to the supplemental methods. In addition, all codesets used to define AUD, COVID-19 positivity, covariates, and outcomes of interest are provided in Supplementary Table 2.
Primary exposure
The primary exposure in this study was AUD. Subjects were considered to have an AUD if they had either 1) a documented diagnosis of AUD or 2) an alcohol-related complication. AUD was defined as a diagnosis of alcoholism, AUD, alcohol abuse, or alcohol dependence in the medical record. Alcohol-related complications included: disorder caused by alcohol, alcoholic liver disease, alcoholic polyneuropathy, alcoholic cardiomyopathy, or alcoholic nervous system disease.
Outcomes
Primary outcomes were 1) hospitalization within 14 days of testing positive for SARS-CoV2 and 2) all-cause mortality. All-cause mortality was defined as any reported death or transition to hospice care. Secondary outcomes include a diagnosis of acute kidney injury and the need for invasive mechanical ventilation.
Other covariates
Confounders, which were determined a priori through directed acyclic graphs, clinical expertise, and relevant literature, included age (continuous and categorical), sex, race/ethnicity (non-Hispanic White, Black or African American, Hispanic or Latino, Missing/unknown, or other), smoking status (non-smoker vs. current or former smoker), body mass index (BMI) (continuous and categorical), and the following comorbid conditions: hypertension, diabetes, myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, dementia, chronic pulmonary disease, rheumatologic disease, hemiplegia or paraplegia, renal disease, and HIV/AIDS). Data on the regional location of subjects was also collected.
Statistical Analysis
Analysis was conducted first on the total cohort of patients meeting our inclusion/exclusion criteria and then on the subset of such patients who required hospitalization. Frequencies and percentages or medians and interquartile ranges of all demographic, clinical, and outcome measures within each of these cohorts were calculated. Comparisons of measures between those with and without AUD in the full cohort and those with and without AUD in the hospitalized cohort were made using or chi-squared tests or Wilcoxon rank-sum tests. A multiple logistic regression model was used to evaluate risk of hospitalization between those with and without AUD in the full cohort while controlling for age, sex, race/ethnicity, smoking, BMI, hypertension, diabetes, myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, dementia, chronic pulmonary disease, rheumatologic disease, hemiplegia or paraplegia, renal disease, and HIV/AIDS. A similar logistic regression model was used to compare all-cause mortality rates between those with and without AUD, first overall, and then limited to those within the hospitalized cohort. The logistic regression models included individual comorbidities as opposed to a composite index such as the Charlson Comorbidity index to avoid including disease states that may lie in the casual pathway between AUD and outcomes of interest. A p-value of <0.05 was considered statistically significant.
RESULTS
Geographic distribution of the cohort
The cohort is evenly distributed within the continental United States. The Northeast had 21% of subjects, Midwest 26%, West 16%, and South 21%, while 15% had missing geographic data (Figure 1).
Figure 1:
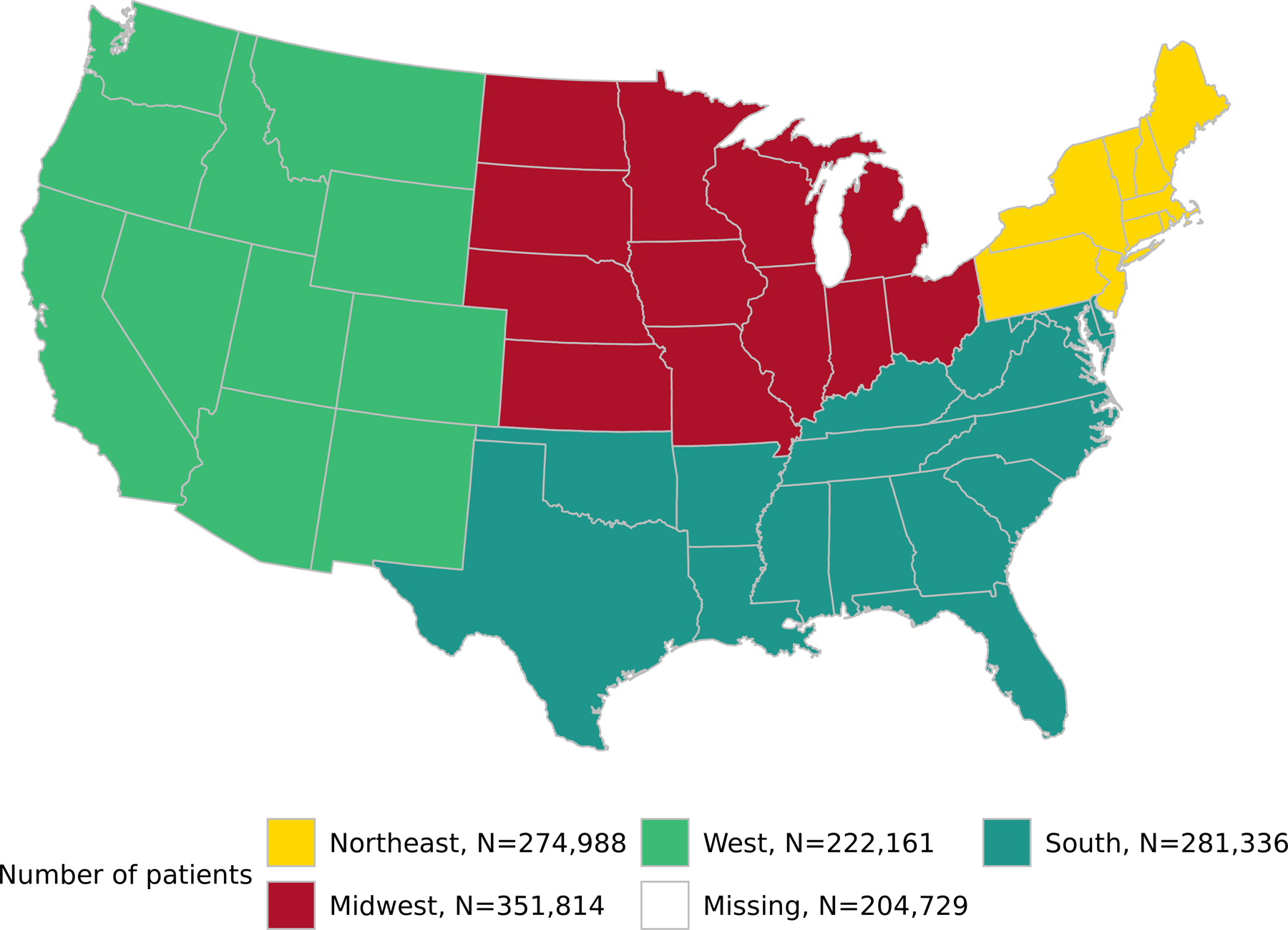
Geographic distribution of the cohort.
Demographics of the overall cohort
After our inclusion and exclusion criteria were satisfied, there were 1,335,028 COVID-19 patients. We identified 25,583 (1.9%) participants with a history of AUD or medical complications of alcohol use, and 1,309,445 without. Their characteristics are summarized in Table 1.
Table 1:
Baseline Characteristics of All COVID-19 Patients with and without Alcohol Use Disorder or Alcohol Complications
|
No AUD or alcohol complication n=1,309,445* |
AUD or Alcohol complication n=25,583* |
p value** | |
|---|---|---|---|
| Age, Median (IQR) | 47 (33, 61) | 51 (38, 62) | <0.001 |
| Age Group | <0.001 | ||
| <29 | 249,220 (19%) | 3,068 (12%) | |
| 30–49 | 463,697 (35%) | 8,671 (34%) | |
| 50–64 | 341,367 (26%) | 8,918 (35%) | |
| >=65 | 255,161 (19%) | 4,926 (19%) | |
| Male Sex | 571,884 (44%) | 16,709 (65%) | <0.001 |
| Race/Ethnicity | <0.001 | ||
| Non-Hispanic White | 671,494 (51%) | 14,302 (56%) | |
| Black or African American | 186,521 (14%) | 4,601 (18%) | |
| Hispanic or Latino | 218,949 (17%) | 4,083 (16%) | |
| Other | 135,715 (10%) | 2,165 (8.5%) | |
| Missing/Unknown | 96,766 (7.4%) | 432 (1.7%) | |
| Current or former smoker | 292,698 (22%) | 9,760 (38%) | <0.001 |
| BMI, Median (IQR) | 29 (25, 34) | 28 (24, 33) | <0.001 |
| BMI Categories | <0.001 | ||
| Underweight (<18.5) | 23,039 (1.8%) | 1,022 (4.0%) | |
| Normal Weight (18.5–24.9) | 213,666 (16%) | 6,427 (25%) | |
| Overweight (25.0–29.9) | 282,861 (22%) | 7,522 (29%) | |
| Obese (>=30.0) | 428,757 (33%) | 9,093 (36%) | |
| Unknown/Missing | 361,122 (28%) | 1,519 (5.9%) | |
| Comorbidities | |||
| Hypertension | 317,401 (24%) | 13,691 (54%) | <0.001 |
| Diabetes Mellitus | 169,737 (13%) | 6,590 (26%) | <0.001 |
| Myocardial Infarction | 24,828 (1.9%) | 1,679 (6.6%) | <0.001 |
| Congestive Heart Failure | 49,445 (3.8%) | 3,070 (12%) | <0.001 |
| Peripheral Vascular Disease | 60,277 (4.6%) | 3,197 (12%) | <0.001 |
| Stroke | 47,860 (3.7%) | 2,779 (11%) | <0.001 |
| Dementia | 13,361 (1.0%) | 819 (3.2%) | <0.001 |
| Chronic Pulmonary Disease | 149,024 (11%) | 6,832 (27%) | <0.001 |
| Rheumatologic Disease | 40,694 (3.1%) | 1,219 (4.8%) | <0.001 |
| Peptic Ulcer Disease | 9,839 (0.8%) | 1,103 (4.3%) | <0.001 |
| Cirrhosis | 7,241 (0.6%) | 3,354 (13%) | <0.001 |
| Hemiplegia or Paraplegia | 6,851 (0.5%) | 463 (1.8%) | <0.001 |
| Renal Disease | 63,104 (4.8%) | 2,932 (11%) | <0.001 |
| Any malignancy (except skin) | 70,030 (5.3%) | 2,420 (9.5%) | <0.001 |
| Metastatic solid tumor | 12,567 (1.0%) | 491 (1.9%) | <0.001 |
| HIV/AIDS | 7,464 (0.6%) | 618 (2.4%) | <0.001 |
| Multiple Comorbidities | 495,307 (38%) | 19,117 (75%) | <0.001 |
| Outcomes | |||
| Hospitalized After COVID Diagnosis | 196,257 (15%) | 6,669 (26%) | <0.001 |
| Death or Transfer to Hospice | 30,533 (2.3%) | 1,264 (4.9%) | <0.001 |
Median (IQR) or n (%)
Wilcoxon rank sum test; Pearson’s Chi-squared test
The percentage of people aged 65 or older was the same (19%) in those with and without AUD. However, there were more middle-aged people (50–64 yrs) in the AUD group (35% vs. 26%). There were more males (65%) in the AUD group compared to the No AUD group (44%). In terms of race and ethnicity, there were more non-Hispanic whites (56% vs 51%) in the AUD group compared to the No AUD group. A higher number of those with AUDs were former or current smokers (38%) compared to those without AUD (22%). Those with AUD had similar rates of obesity (33%) as those without (36%). Those with AUD, however, were more likely to be underweight (4% vs. 1.8%), normal weight (25% vs. 16%) and overweight (29% vs. 22%) compared to those without AUD. There were higher levels of “unknown” BMI in those without AUD (Table 1).
COVID-19 patients with AUD were more likely to be admitted to the hospital
COVID-19 patients with AUD were more likely to be admitted to the hospital (26%) compared to those without AUD (15%) (p<0.001). Those with AUD were also twice as likely to die or be transitioned to hospice care (4.9% vs.2.3%, p<0.001) (Table 1). The unadjusted odds ratio for hospitalization for those with AUD vs those without was 2.00 (CI 1.94–2.06, p<0.001) (Table 2a). After adjusting for age, sex, race/ethnicity, smoking, BMI, hypertension, diabetes, myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, dementia, chronic pulmonary disease, rheumatologic disease, hemiplegia or paraplegia, renal disease, and HIV/AIDS, those with an AUD still had higher odds of requiring hospitalization (OR=1.51, 95% CI 1.46–1.56, p<0.001) (Table 2b).
Table 2:
Association of Alcohol Use Disorder and Hospitalization in COVID-19 Positive Cohort
| A. Unadjusted Odds Ratio for Hospitalization | |||
|---|---|---|---|
| Odds Ratio | Confidence Interval | p value | |
| History of AUD or Alcohol Complications | 2.00 | 1.94, 2.06 | <0.001 |
| B. Adjusted Odds Ratio for Hospitalization | |||
| Odds Ratio | Confidence Interval | p value | |
| Age | 1.04 | 1.04, 1.04 | <0.001 |
| Male Sex | 1.37 | 1.35, 1.38 | <0.001 |
| Race/Ethnicity | |||
| Non-Hispanic White (reference) | |||
| Black or African American | 2.21 | 2.18, 2.24 | <0.001 |
| Hispanic or Latino | 1.90 | 1.87, 1.93 | <0.001 |
| Missing/Unknown | 0.87 | 0.85, 0.90 | <0.001 |
| Other | 1.68 | 1.65, 1.71 | <0.001 |
| Smoking Status | |||
| Non-smoker (reference) | |||
| Current or Former | 1.11 | 1.09, 1.12 | <0.001 |
| Body Mass Index (BMI) | |||
| <18.5 | 1.14 | 1.10, 1.18 | <0.001 |
| 18.5–24.9 (reference) | |||
| 25–29.9 | 1.00 | 0.99, 1.02 | 0.9 |
| >=30 | 1.34 | 1.32, 1.36 | <0.001 |
| Missing/Unknown | 0.31 | 0.31, 0.32 | <0.001 |
| Comorbidities | |||
| Hypertension | 0.65 | 0.64, 0.66 | <0.001 |
| Diabetes | 1.01 | 1.00, 1.03 | 0.061 |
| Myocardial Infarction | 1.32 | 1.28, 1.36 | <0.001 |
| Congestive Heart Failure | 1.84 | 1.80, 1.88 | <0.001 |
| Peripheral Vascular Disease | 1.01 | 0.99, 1.03 | 0.2 |
| Stroke | 1.16 | 1.14, 1.19 | <0.001 |
| Dementia | 1.76 | 1.69, 1.82 | <0.001 |
| Chronic Pulmonary Disease | 0.91 | 0.89, 0.92 | <0.001 |
| Rheumatologic Disease | 0.79 | 0.77, 0.81 | <0.001 |
| Hemiplegia or Paraplegia | 2.05 | 1.94, 2.16 | <0.001 |
| Renal Disease | 1.70 | 1.66, 1.73 | <0.001 |
| HIV/AIDS | 0.68 | 0.64, 0.72 | <0.001 |
| Adjusted Odds ratio for Hospitalization for those with AUD or alcohol complication | 1.51 | 1.46, 1.56 | <0.001 |
COVID-19 patients with AUDs had higher all-cause mortality
In the full cohort, the crude odds ratio for all-cause mortality in the full cohort for AUD vs. no AUD was 2.18 (CI 2.05–2.31, p<0.001) (Table 3a). After adjusting for age, sex, race/ethnicity, smoking, BMI, hypertension, diabetes, myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, dementia, chronic pulmonary disease, rheumatologic disease, hemiplegia or paraplegia, renal disease, and HIV/AIDS, those with an AUD still had higher odds of mortality or transfer to hospice of (OR=1.55, 95% CI 1.46–1.65, p<0.001) (Table 3b).
Table 3:
Death or Transfer to Hospice in the COVID-19 Positive Cohort
| A. Unadjusted Odds Ratio for Death or Transfer to Hospice | |||
|---|---|---|---|
| Covariate | Odds Ratio | Confidence Interval | p value |
| History of AUD or Alcohol Complications | 2.18 | 2.05, 2.31 | <0.001 |
| B. Adjusted Odds Ratio for Death or Transfer to Hospice | |||
| Covariate | Odds Ratio | Confidence Interval | p value |
| Age | 1.08 | 1.08, 1.08 | <0.001 |
| Male Sex | 1.68 | 1.64, 1.72 | <0.001 |
| Race/Ethnicity | |||
| Non-Hispanic White (reference) | |||
| Black or African American | 1.54 | 1.49, 1.59 | <0.001 |
| Hispanic or Latino | 1.54 | 1.49, 1.60 | <0.001 |
| Missing/Unknown | 1.03 | 0.96, 1.10 | 0.4 |
| Other | 1.47 | 1.42, 1.53 | <0.001 |
| Smoking Status | |||
| Non-smoker (reference) | |||
| Current or Former | 1.11 | 1.08, 1.14 | <0.001 |
| Body Mass Index (BMI) | |||
| <18.5 | 1.46 | 1.37, 1.56 | <0.001 |
| 18.5–24.9 (reference) | |||
| 25–29.9 | 0.82 | 0.80, 0.85 | <0.001 |
| >=30 | 1.10 | 1.06, 1.13 | <0.001 |
| Missing/Unknown | 0.45 | 0.43, 0.48 | <0.001 |
| Comorbidities | |||
| Hypertension | 0.66 | 0.64, 0.68 | <0.001 |
| Diabetes | 1.14 | 1.10, 1.17 | <0.001 |
| Myocardial Infarction | 1.18 | 1.12, 1.23 | <0.001 |
| Congestive Heart Failure | 1.81 | 1.74, 1.87 | <0.001 |
| Peripheral Vascular Disease | 1.07 | 1.03, 1.11 | <0.001 |
| Stroke | 1.14 | 1.09, 1.18 | <0.001 |
| Dementia | 1.96 | 1.87, 2.05 | <0.001 |
| Chronic Pulmonary Disease | 1.08 | 1.05, 1.11 | <0.001 |
| Hemiplegia or Paraplegia | 1.91 | 1.76, 2.08 | <0.001 |
| Renal Disease | 1.80 | 1.74, 1.86 | <0.001 |
| HIV/AIDS | 0.95 | 0.82, 1.08 | 0.4 |
| Adjusted Odds ratio for death or transfer to hospice for those with a history of AUD or Alcohol Complications | 1.55 | 1.46, 1.65 | <0.001 |
COVID-19 in cohort of Hospitalized patients
We investigated outcomes in hospitalized patients alone because the most reliable and timely death data are available in EHRs of hospitalized patients.
Demographics of hospitalized COVID-19 patients with and without AUDs
Demographic, clinical, and outcome statistics for the COVID-19 patients who were hospitalized are presented in Table 4. Those with AUD were younger, with only 28% being over the age of 65 compared to 40% in those without AUD. The majority were male (72%), compared to those without AUDs (50%). Those with AUD were more likely to be non-Hispanic whites (51%) versus those without AUD (45%). Those with AUD were more likely to be underweight and normal weight than those without AUD. Rates of obesity were lower in those with AUD (35%) compared to those without AUD (46%). Those with AUD were more likely to have multiple comorbidities (84%) than those without AUD (49%). As expected, those with AUD were more frequently diagnosed with cirrhosis (22%) than those without (1.5%) (Table 4).
Table 4:
Demographics of Hospitalized COVID-19 Patients with and without AUD
|
No AUD or alcohol complication n= 196,2571 |
AUD or Alcohol complication n=6,6691 |
p value2 | |
|---|---|---|---|
| Age, Median (IQR) | 60 (44, 72) | 57 (44, 66) | <0.001 |
| Age Group | |||
| <29 | 16963 (8.6%) | 412 (6.2%) | <0.001 |
| 30–49 | 45618 (23%) | 1831 (27%) | |
| 50–64 | 54844 (28%) | 2572 (39%) | |
| >=65 | 78832 (40%) | 1854 (28%) | |
| Male Sex | 97510 (50%) | 4787 (72%) | <0.001 |
| Race/Ethnicity | <0.001 | ||
| Non-Hispanic White | 88789 (45%) | 3372 (51%) | |
| Black or African American | 41955 (21%) | 1537 (23%) | |
| Hispanic or Latino | 37648 (19%) | 1064 (16%) | |
| Other | 22123 (11%) | 596 (8.9%) | |
| Missing/Unknown | 5742 (2.9%) | 100 (1.5%) | |
| Current or former smoker | 54983 (28%) | 2883 (43%) | <0.001 |
| BMI Categories | <0.001 | ||
| Underweight (<18.5) | 5074 (2.6%) | 386 (5.8%) | |
| Normal Weight (18.5–24.9) | 33031 (17%) | 1880 (28%) | |
| Overweight (25.0–29.9) | 50377 (26%) | 1848 (28%) | |
| Obese (>=30.0) | 89540 (46%) | 2327 (35%) | |
| Unknown/Missing | 18235 (9.3%) | 228 (3.4%) | |
| Individual Comorbidities | |||
| Hypertension | 71045 (36%) | 4383 (66%) | <0.001 |
| Diabetes Mellitus | 43925 (22%) | 2281 (34%) | <0.001 |
| Myocardial Infarction | 9858 (5.0%) | 777 (12%) | <0.001 |
| Congestive Heart Failure | 21165 (11%) | 1435 (22%) | <0.001 |
| Peripheral Vascular Disease | 17811 (9.1%) | 1240 (19%) | <0.001 |
| Stroke | 16024 (8.2%) | 1127 (17%) | <0.001 |
| Dementia | 6476 (3.3%) | 421 (6.3%) | <0.001 |
| Chronic Pulmonary Disease | 29264 (15%) | 2147 (32%) | <0.001 |
| Rheumatologic Disease | 7496 (3.8%) | 347 (5.2%) | <0.001 |
| Peptic Ulcer Disease | 2635 (1.3%) | 427 (6.4%) | <0.001 |
| Cirrhosis | 2968 (1.5%) | 1448 (22%) | <0.001 |
| Hemiplegia or Paraplegia | 2880 (1.5%) | 197 (3.0%) | <0.001 |
| Renal Disease | 24650 (13%) | 1352 (20%) | <0.001 |
| Any malignancy (except skin) | 16931 (8.6%) | 846 (13%) | <0.001 |
| Metastatic solid tumor | 3828 (2.0%) | 210 (3.1%) | <0.001 |
| HIV/AIDS | 1188 (0.6%) | 160 (2.4%) | <0.001 |
| Multiple Comorbidities | 97017 (49%) | 5633 (84%) | <0.001 |
| Outcomes | |||
| Acute Kidney Injury | 32630 (17%) | 1232 (18%) | <0.001 |
| Invasive Mechanical Ventilation | 18728 (9.5%) | 609 (9.1%) | 0.3 |
| Death or Transfer to Hospice | 23397 (12%) | 878 (13%) | 0.002 |
| Inpatient therapies | |||
| Steroids | 39220 (20%) | 1011 (15%) | <0.001 |
| Remdesivir | 5246 (2.7%) | 265 (4.0%) | <0.001 |
| Transfusion | 15062 (7.7%) | 497 (7.5%) | 0.5 |
| Vasopressor | 39220 (20%) | 1011 (15%) | <0.001 |
Median (IQR); n (%)
Wilcoxon rank sum test; Pearson’s Chi-squared test
Hospitalized COVID-19 patients with AUDs had higher all-cause mortality than those without AUDs
In unadjusted comparisons, COVID-19 patients with AUD were somewhat more likely to develop acute kidney injury (18%) compared to those without AUD (17%) (p<0.001). The two groups had similar rates of requiring invasive mechanical ventilation (9.5 % versus 9.1% (p=0.3)). Those with AUD also had somewhat higher rates of death or transfer to hospice care (13%) compared to those without AUD (12%) (p=0.002) (Table 4). The rates of discharge to hospice were not significantly different between those with AUD (0.7%) and without AUD (0.6%).
The crude odds ratio for death or transfer to hospice for AUD vs. no AUD was 1.12 (CI 1.04–1.20, p=0.002) (Table 5A). After adjusting for all relevant confounders as previously described, those with AUD or alcohol complications had 15% higher odds of all-cause mortality, with an odds ratio of 1.15 (95% CI 1,106–1.24, p<0.001) (Table 5B).
Table 5:
Association of Alcohol Use Disorder and Inpatient Death or Transfer to Hospice in Hospitalized COVID-19 Positive Cohort
| A. Unadjusted Odds Ratio for Inpatient Death or Transfer to Hospice | |||
|---|---|---|---|
| Covariate | Odds Ratio | Confidence Interval | p value |
| History of AUD or Alcohol Complications | 1.12 | 1.04, 1.20 | 0.002 |
| B. Adjusted Odds Ratio for Inpatient Death or Transfer to Hospice | |||
| Covariate | Odds Ratio | Confidence Interval | p value |
| Age | 1.05 | 1.05, 1.05 | <0.001 |
| Male Sex | 1.47 | 1.42, 1.51 | <0.001 |
| Race/Ethnicity | |||
| Non-Hispanic White (reference) | |||
| Black or African American | 1.03 | 0.99, 1.07 | 0.2 |
| Hispanic or Latino | 1.11 | 1.06, 1.15 | <0.001 |
| Missing/Unknown | 1.41 | 1.30, 1.54 | <0.001 |
| Other | 1.07 | 1.02, 1.12 | 0.008 |
| Smoking Status | |||
| Non-smoker (reference) | |||
| Current or Former | 1.14 | 1.10, 1.17 | <0.001 |
| Body Mass Index (BMI) | |||
| <18.5 | 1.23 | 1.14, 1.34 | <0.001 |
| 18.5–24.9 (reference) | |||
| 25–29.9 | 0.89 | 0.85, 0.93 | <0.001 |
| >=30 | 1.04 | 1.00, 1.09 | 0.030 |
| Missing/Unknown | 0.86 | 0.81, 0.91 | <0.001 |
| Comorbidities | |||
| Hypertension | 0.81 | 0.78, 0.85 | <0.001 |
| Diabetes | 1.06 | 1.03, 1.10 | 0.001 |
| Myocardial Infarction | 1.04 | 0.98, 1.10 | 0.2 |
| Congestive Heart Failure | 1.38 | 1.33, 1.45 | <0.001 |
| Peripheral Vascular Disease | 1.07 | 1.02, 1.11 | 0.005 |
| Stroke | 1.09 | 1.04, 1.14 | <0.001 |
| Dementia | 1.40 | 1.32, 1.48 | <0.001 |
| Chronic Pulmonary Disease | 1.05 | 1.01, 1.10 | 0.010 |
| Rheumatologic Disease | 0.96 | 0.89, 1.03 | 0.2 |
| Hemiplegia or Paraplegia | 1.41 | 1.28, 1.55 | <0.001 |
| Renal Disease | 1.47 | 1.41, 1.53 | <0.001 |
| HIV/AIDS | 0.96 | 0.79, 1.14 | 0.6 |
| Adjusted Odds Ratio for inpatient mortality or transfer to hospice for those with a history of AUD or Alcohol Complications | 1.15 | 1.06, 1.24 | <0.001 |
DISCUSSION
In this analysis, we have demonstrated that COVID-19 patients with an AUD documented in the medical record are more likely to be hospitalized for COVID-19 and had a higher all-cause mortality than patients without an AUD. One of the strengths of this analysis was that we were able to use a large, multicenter database that included 44 centers. This analysis included more than one million cases of COVID-19 throughout the United States. It includes centers from rural areas as well as urban. Temporally, it includes patients not only from the initial surge, but also included patients from later stages of the pandemic.
In this cohort, those with AUD had higher rates of several comorbidities. Because of this, we corrected for comorbidities in our analysis. Those with AUD had higher rates of cirrhosis. A small, multicenter trial reported that cirrhosis patients with COVID-19 (n=37) had a 30% mortality, similar to those hospitalized with cirrhosis alone (20% mortality), but a higher mortality than those with COVID-19 alone (13% mortality). Like our findings, the cirrhosis groups had significantly higher current alcohol use (10–25%) compared to subjects with COVID-19 alone (2%)(Bajaj et al., 2021). It is likely that both cirrhosis and AUD affect the severity of COVID-19. Likewise, those with AUD had higher rates of chronic pulmonary diseases, likely due to their higher rates of smoking (Table 1). Chronic Obstructive Pulmonary Disease (COPD) is thought to increase severity and mortality in COVID-19 pneumonia (Lippi and Henry, 2020, Wu et al., 2020). In the N3C database the risk of death was 2 times higher in those with COPD (Meza et al., 2021). Early in the pandemic, it was reported that hypertension was a very common comorbidity with COVID-19 (Guan et al., 2020). There is evidence that patients with hypertension may have more severe outcomes with COVID-19 infection, including worse mortality (Roncon et al., 2020, Pranata et al., 2020a, Zuin et al., 2020) (Du et al., 2021). Likewise, diabetes is an important comorbidity that leads to worse outcomes in those with COVID-19 (Singh et al., 2020). This is true for both Type I and Type II Diabetes (Barron et al., 2020).
Having an AUD is a known risk factor for developing acute respiratory distress syndrome (ARDS) (Moss et al., 2003), a severe complication of COVID-19. AUD is also a risk factor for developing multisystem failure with ARDS (Moss and Burnham, 2003). Unfortunately, in this cohort we were unable to determine whether ARDS was increased in those with AUD due to widely variable ways of defining ARDS at the different centers. This is an important topic that needs further study.
In this study, we report higher hospitalization rates in those with AUD. Our results are in contrast to a smaller UK study of 750 COVID-19 cases that showed that heavy alcohol consumption was not related to COVID-19 hospitalization (Hamer et al., 2020). However, interestingly, the reference group for this study was those with “moderate” alcohol intake (defined as ≤3 drinks per day for men and ≤2 drinks per day for women), rather than the never drinkers, which may have affected the analysis.
Our analysis has several limitations that must be taken into consideration. A major limitation of this analysis was that Alcohol Use Disorder Identification Test (AUDIT) scores, and Short Michigan Alcohol Screening tests (SMAST) were not available. Likewise, we were unable to accurately quantify how many standard drinks each subject was drinking per day or per occasion prior to, or at the time of COVID-19 diagnosis. Unfortunately, alcohol use was not well documented in the medical records of most participating sites. Because of this, we had to rely on a diagnosis of AUD or alcohol-related complications. It is well known that AUDs are underreported in the medical record (Gryczynski et al., 2020). This means that we have likely only studied only those with very severe AUD. In addition, our “No AUD” group likely includes individuals who have AUD that are not documented. It is known that those who drink even 2–3 drinks a day have a higher risk of ARDS (Simou et al., 2018b), and we were unable to assess this in this cohort.
An interesting issue in alcohol related EHR studies is the association of AUD and cigarette smoking (Falk et al., 2006). Cigarette smoking is twice as common in those with AUD and those without (Weinberger et al., 2019). To address this issue, we have corrected for former or current cigarette smoking in all of our analyses. Due to constraints with the N3C database, we were not able to separately correct for former and current smoking. Early on in the pandemic, there were controversial reports that current smokers were spared from COVID-19 infection (Huang et al., 2020). Since then, the CDC has acknowledged that both former and current smoking are both risk factors for severe disease and the concept has been relegated to a myth (van Westen-Lagerweij et al., 2021, Patanavanich and Glantz, 2020, Berlin and Thomas, 2020, Kaur et al., 2020, Shastri et al., 2021, Hopkinson et al., 2021, Peng et al., 2021, Umnuaypornlert et al., 2021, Patanavanich and Glantz, 2021, Mohsin et al., 2021, Rodgers et al., 2021, Neira et al., 2021, Lowe et al., 2021, Zhang et al., 2021).
Another limitation is that our data suggest that those with a diagnosis of AUD were better known to the health care system. For instance, we had lower numbers of missing or unknown race/ethnicity and BMI in those that had a diagnosis of AUD, which could be due to more frequent clinic visits or hospitalizations prior to testing positive for COVID-19. This may have increased the documentation of comorbidities in those with AUD compared to those without. Of interest, in this cohort of patients with COVID-19, those with AUD had a significantly higher number of medical comorbidities documented. This included liver disease, hypertension, diabetes, history of myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, dementia, chronic pulmonary disease, rheumatologic disease, hemiplegia or paraplegia, and renal disease. Having significant comorbidities is a known risk factor for poor outcomes in COVID-19 (Ejaz et al., 2020, Yang et al., 2020). AUD is known to increase many of these comorbidities such as liver disease (Mandayam et al., 2004), diabetes (Carlsson et al., 2005), stroke (Reynolds et al., 2003), hypertension (Saunders et al., 1981), dementia (Rehm et al., 2019) renal disease (Perneger et al., 1999) and HIV/AIDs (Ferguson et al., 2020). So, it is not clear if the comorbidities are higher or better documented in those with AUD.
Likewise, there are many limitations of our research that are related to the limitations of the N3C database. For example, we could not include site effects or timing of the infection in the analysis due to blinding to site and date shifting in the cohort. However, it is important to note that our cohort was fairly equally distributed throughout the US (Figure 1), and was spread over a period of time that included surges in several geographic areas. Another limitation is the lack of availability of vaccination status. Vaccination is an important tool to prevent hospitalization and death due to COVID-19. Those with substance abuse disorders (including AUD) are known to have more breakthrough infections with COVID-19 (Wang et al., 2022). However, reliable vaccination status was only available in a few sites where the EHR was connected to state health information exchanges or other agencies.
This analysis suggests that those with AUD are more likely to be hospitalized for COVID-19 and have a higher all-cause mortality. It is not clear whether this is simply due to alcohol intake or if this is related to comorbidities caused by heavy alcohol intake, although our adjustment for these conditions in statistical modeling does indicate independent effects of AUD. Further research is needed to determine whether AUD are an independent risk factor for poor outcomes with viral pneumonia such as COVID-19.
However, given the strong association of AUD with the need for hospitalization in COVID-19 pneumonia, clinicians should ask patients about alcohol use and misuse. Those with AUD or alcohol misuse should be carefully observed for deterioration and clinicians should have a low threshold for admission to the hospital and transfer for ICU care if needed.
Supplementary Material
Acknowledgements
The analyses described in this publication were conducted with data or tools accessed through the NCATS N3C Data Enclave (https://COVID-19.cd2h.org) and N3C Attribution & Publication Policy v 1.2–2020-08–25b supported by NCATS U24 TR002306. This research was possible because of the patients whose information is included within the data and the organizations (https://ncats.nih.gov/n3c/resources/data-contribution/data-transfer-agreement-signatories) and scientists who have contributed to the ongoing development of this community resource [https://doi.org/10.1093/jamia/ocaa196].
Funding:
The project described was supported by the National Institute of General Medical Sciences, 5U54GM104942–04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Other support for this project was provided by the NIAAA (R25AA020818 to KLB and R24AA019661 to ELB, TAW, KLB) and the Department of Veterans Affairs (I01CX001714 to CLH). Todd Wyatt is supported by a Research Career Scientist award from the Department of Veterans Affairs (IK6BX003781).
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose.
Bibliography
- BAILEY KL, ROMBERGER DJ, KATAFIASZ DM, HEIRES AJ, SISSON JH, WYATT TA & BURNHAM EL 2015. TLR 2 and TLR 4 Expression and Inflammatory Cytokines are Altered in the Airway Epithelium of Those with Alcohol Use Disorders. Alcoholism: Clinical and Experimental Research, 39, 1691–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILEY KL, SAMUELSON DR & WYATT TA 2021. Alcohol use disorder: A pre-existing condition for COVID-19? Alcohol, 90, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILEY KL, WYATT TA, KATAFIASZ DM, TAYLOR KW, HEIRES AJ, SISSON JH, ROMBERGER DJ & BURNHAM EL 2019. Alcohol and cannabis use alter pulmonary innate immunity. Alcohol, 80, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAJAJ JS, GARCIA-TSAO G, BIGGINS SW, KAMATH PS, WONG F, MCGEORGE S, SHAW J, PEARSON M, CHEW M & FAGAN A 2021. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut, 70, 531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRON E, BAKHAI C, KAR P, WEAVER A, BRADLEY D, ISMAIL H, KNIGHTON P, HOLMAN N, KHUNTI K & SATTAR N 2020. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. The lancet Diabetes & endocrinology, 8, 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERLIN I & THOMAS D 2020. Does Smoking Protect against Being Hospitalized for COVID-19? International journal of environmental research and public health, 17, 9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIAN J, LYU T, LOIACONO A, VIRAMONTES TM, LIPORI G, GUO Y, WU Y, PROSPERI M, GEORGE JR TJ & HARLE CA 2020. Assessing the practice of data quality evaluation in a national clinical data research network through a systematic scoping review in the era of real-world data. Journal of the American Medical Informatics Association, 27, 1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNHAM EL, KOVACS EJ & DAVIS CS 2013. Pulmonary cytokine composition differs in the setting of alcohol use disorders and cigarette smoking. American Journal of Physiology-Lung Cellular and Molecular Physiology, 304, L873–L882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALESNICK B & VERNICK H 1971. Antitussive activity of ethanol. Quarterly journal of studies on alcohol, 32, 434–441. [PubMed] [Google Scholar]
- CARLSSON S, HAMMAR N & GRILL V 2005. Alcohol consumption and type 2 diabetes. Diabetologia, 48, 1051–1054. [DOI] [PubMed] [Google Scholar]
- CHANG MC, PARK Y-K, KIM B-O & PARK D 2020. Risk factors for disease progression in COVID-19 patients. BMC infectious diseases, 20, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON BE, WEN C, FRENCH T, WILLIAMS JL, DUKE JD & GRANNIS SJ 2020. Extending an open-source tool to measure data quality: case report on Observational Health Data Science and Informatics (OHDSI). BMJ health & care informatics, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONG E, DU H, & GARDNER L 2020. An interactive web-based dashboard to track COVID-19 in real time. The Lancet. Infectious diseases, 20, 533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DU Y, ZHOU N, ZHA W & LV Y 2021. Hypertension is a clinically important risk factor for critical illness and mortality in COVID-19: A meta-analysis. Nutrition, Metabolism and Cardiovascular Diseases, 31, 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EJAZ H, ALSRHANI A, ZAFAR A, JAVED H, JUNAID K, ABDALLA AE, ABOSALIF KO, AHMED Z & YOUNAS S 2020. COVID-19 and comorbidities: Deleterious impact on infected patients. Journal of infection and public health [DOI] [PMC free article] [PubMed]
- ESPOSITO AJ, MENON AA, GHOSH AJ, PUTMAN RK, FREDENBURGH LE, EL-CHEMALY SY, GOLDBERG HJ, BARON RM, HUNNINGHAKE GM & DOYLE TJ 2020. Increased odds of death for patients with interstitial lung disease and COVID-19: a case–control study. American Journal of Respiratory and Critical Care Medicine, 202, 1710–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALK DE, YI H-Y & HILLER-STURMHÖFEL S 2006. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism, 29, 162–171. [PMC free article] [PubMed] [Google Scholar]
- FERGUSON TF, THEALL KP, BRASHEAR M, MAFFEI V, BEAUCHAMP A, SIGGINS RW, SIMON L, MERCANTE D, NELSON S, WELSH DA & MOLINA PE 2020. Comprehensive Assessment of Alcohol Consumption in People Living with HIV (PLWH): The New Orleans Alcohol Use in HIV Study. Alcohol Clin Exp Res, 44, 1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO YD, DING M, DONG X, ZHANG JJ, KURSAT AZKUR A, AZKUR D, GAN H, SUN YL, FU W & LI W 2021. Risk factors for severe and critically ill COVID‐19 patients: a review. Allergy, 76, 428–455. [DOI] [PubMed] [Google Scholar]
- GRYCZYNSKI J, NORDECK CD, MARTIN RD, WELSH C, SCHWARTZ RP, MITCHELL SG & JAFFE JH 2020. Leveraging health information exchange for clinical research: extreme underreporting of hospital service utilization among patients with substance use disorders. Drug and alcohol dependence, 212, 107992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUAN W-J, NI Z-Y, HU Y, LIANG W-H, OU C-Q, HE J-X, LIU L, SHAN H, LEI C-L & HUI DS 2020. Clinical characteristics of coronavirus disease 2019 in China. New England journal of medicine, 382, 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAENDEL MA, CHUTE CG, BENNETT TD, EICHMANN DA, GUINNEY J, KIBBE WA, PAYNE PR, PFAFF ER, ROBINSON PN & SALTZ JH 2021. The National COVID Cohort Collaborative (N3C): rationale, design, infrastructure, and deployment. Journal of the American Medical Informatics Association, 28, 427–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMER M, KIVIMÄKI M, GALE CR & BATTY GD 2020. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: A community-based cohort study of 387,109 adults in UK. Brain, Behavior, and Immunity, 87, 184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPKINSON NS, ROSSI N, EL-SAYED_MOUSTAFA J, LAVERTY AA, QUINT JK, FREIDIN M, VISCONTI A, MURRAY B, MODAT M & OURSELIN S 2021. Current smoking and COVID-19 risk: results from a population symptom app in over 2.4 million people. Thorax, 76, 714–722. [DOI] [PubMed] [Google Scholar]
- HUANG C, WANG Y, LI X, REN L, ZHAO J, HU Y, ZHANG L, FAN G, XU J & GU X 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet, 395, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JERRELLS TR, PAVLIK JA, DEVASURE J, VIDLAK D, COSTELLO A, STRACHOTA JM & WYATT TA 2007. Association of chronic alcohol consumption and increased susceptibility to and pathogenic effects of pulmonary infection with respiratory syncytial virus in mice. Alcohol, 41, 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN RE, ADAB P & CHENG K 2020. Covid-19: risk factors for severe disease and death British Medical Journal Publishing Group. [DOI] [PubMed] [Google Scholar]
- KAUR G, LUNGARELLA G & RAHMAN I 2020. SARS-CoV-2 COVID-19 susceptibility and lung inflammatory storm by smoking and vaping. Journal of Inflammation, 17, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNIGHT SR, HO A, PIUS R, BUCHAN I, CARSON G, DRAKE TM, DUNNING J, FAIRFIELD CJ, GAMBLE C & GREEN CA 2020. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. bmj, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI, XU S, YU M, WANG K, TAO Y, ZHOU Y, SHI J, ZHOU M, WU B, YANG Z, ZHANG C, YUE J, ZHANG Z, RENZ H, LIU X, XIE J, XIE M & ZHAO J 2020. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. Journal of Allergy and Clinical Immunology, 146, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIPPI G & HENRY BM 2020. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respiratory medicine, 167, 105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWE KE, ZEIN J, HATIPOĞLU U & ATTAWAY A 2021. Association of smoking and cumulative pack-year exposure with COVID-19 outcomes in the Cleveland Clinic COVID-19 Registry. JAMA internal medicine, 181, 709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDAYAM S, JAMAL MM & MORGAN TR Epidemiology of alcoholic liver disease. Seminars in liver disease, 2004. Published in 2004 by Thieme Medical Publishers, Inc., 333 Seventh Avenue …, 217–232. [DOI] [PubMed]
- MARINI JJ & GATTINONI L 2020. Management of COVID-19 Respiratory Distress. JAMA, 323, 2329–2330. [DOI] [PubMed] [Google Scholar]
- MEYERHOLZ DK, EDSEN-MOORE M, MCGILL J, COLEMAN RA, COOK RT & LEGGE KL 2008. Chronic alcohol consumption increases the severity of murine influenza virus infections. The Journal of Immunology, 181, 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEZA D, KHUDER B, BAILEY JI, ROSENBERG SR, KALHAN R & REYFMAN PA 2021. Mortality from COVID-19 in Patients with COPD: A US Study in the N3C Data Enclave. International Journal of Chronic Obstructive Pulmonary Disease, 16, 2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOHSIN FM, TONMON TT, NAHRIN R, TITHY SA, AME FA, ARA I, ALAM ST, PERVEJ AMA, SHAHJALAL M & HAWLADER MDH 2021. Association Between Smoking and COVID-19 Severity: Evidence from Bangladesh. Journal of multidisciplinary healthcare, 14, 1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSS M & BURNHAM EL 2003. Chronic alcohol abuse, acute respiratory distress syndrome, and multiple organ dysfunction. Crit Care Med, 31, S207–12. [DOI] [PubMed] [Google Scholar]
- MOSS M & BURNHAM EL 2006. Alcohol abuse in the critically ill patient. Lancet, 368, 2231–42. [DOI] [PubMed] [Google Scholar]
- MOSS M, PARSONS PE, STEINBERG KP, HUDSON LD, GUIDOT DM, BURNHAM EL, EATON S & COTSONIS GA 2003. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Critical care medicine, 31, 869–877. [DOI] [PubMed] [Google Scholar]
- MUELLER AL, MCNAMARA MS & SINCLAIR DA 2020. Why does COVID-19 disproportionately affect older people? Aging, 12, 9959–9981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIRA DP, WATTS A, SEASHORE J, POLYCHRONOPOULOU E, KUO Y-F & SHARMA G 2021. Smoking and risk of COVID-19 hospitalization. Respiratory medicine, 182, 106414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORGANIZATION, W. H. 2019. Global status report on alcohol and health 2018, World Health Organization. [Google Scholar]
- PATANAVANICH R & GLANTZ SA 2020. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine and Tobacco Research, 22, 1653–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATANAVANICH R & GLANTZ SA 2021. Smoking is associated with worse outcomes of COVID-19 particularly among younger adults: a systematic review and meta-analysis. BMC Public Health, 21, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECKHAM H, DE GRUIJTER NM, RAINE C, RADZISZEWSKA A, CIURTIN C, WEDDERBURN LR, ROSSER EC, WEBB K & DEAKIN CT 2020. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nature Communications, 11, 6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENG F, LEI S, ZHANG Q, ZHONG Y & WU S 2021. Smoking Is Correlated With the Prognosis of Coronavirus Disease 2019 (COVID-19) Patients: An Observational Study. Frontiers in physiology, 12, 634842–634842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERNEGER TV, WHELTON PK, PUDDEY IB & KLAG MJ 1999. Risk of end-stage renal disease associated with alcohol consumption. American journal of epidemiology, 150, 1275–1281. [DOI] [PubMed] [Google Scholar]
- POPKIN BM, DU S, GREEN WD, BECK MA, ALGAITH T, HERBST CH, ALSUKAIT RF, ALLUHIDAN M, ALAZEMI N & SHEKAR M 2020. Individuals with obesity and COVID‐19: A global perspective on the epidemiology and biological relationships. Obesity Reviews, 21, e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRANATA R, LIM MA, HUANG I, RAHARJO SB & LUKITO AA 2020a. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: A systematic review, meta-analysis and meta-regression. Journal of the renin-angiotensin-aldosterone system : JRAAS, 21, 1470320320926899–1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRANATA R, SOEROTO A, HUANG I, LIM M, SANTOSO P, PERMANA H & LUKITO A 2020b. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. The International Journal of Tuberculosis and Lung Disease, 24, 838–843. [DOI] [PubMed] [Google Scholar]
- REHM J, HASAN OS, BLACK SE, SHIELD KD & SCHWARZINGER M 2019. Alcohol use and dementia: a systematic scoping review. Alzheimer’s research & therapy, 11, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS K, LEWIS B, NOLEN JDL, KINNEY GL, SATHYA B & HE J 2003. Alcohol consumption and risk of stroke: a meta-analysis. Jama, 289, 579–588. [DOI] [PubMed] [Google Scholar]
- RODGERS A, NADKARNI M, INDREBERG EK, ALFALLAJ L & KABIR Z 2021. Smoking and COVID-19: a literature review of cohort studies in non-Chinese population settings. Tobacco use insights, 14, 1179173X20988671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RONCON L, ZUIN M, ZULIANI G & RIGATELLI G 2020. Patients with arterial hypertension and COVID-19 are at higher risk of ICU admission. British journal of anaesthesia, 125, e254–e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAENGOW U, ASSANANGKORNCHAI S & CASSWELL S 2021. Alcohol: a probable risk factor of COVID-19 severity. Addiction, 116, 204–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAUNDERS J, BEEVERS D & PATON A 1981. Alcohol-induced hypertension. The Lancet, 318, 653–656. [DOI] [PubMed] [Google Scholar]
- SHASTRI MD, SHUKLA SD, CHONG WC, KC R, DUA K, PATEL RP, PETERSON GM & O’TOOLE RF 2021. Smoking and COVID-19: What we know so far. Respiratory Medicine, 176, 106237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMET S, WYATT T, PAVLIK J & SISSON J 2013. Alcohol-induced tight junction dysfunction primes airways for RSV infection. Alcohol, 47, 575. [Google Scholar]
- SIMOU E, BRITTON J & LEONARDI-BEE J 2018a. Alcohol and the risk of pneumonia: a systematic review and meta-analysis. BMJ open, 8, e022344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMOU E, LEONARDI-BEE J & BRITTON J 2018b. The effect of alcohol consumption on the risk of ARDS: a systematic review and meta-analysis. Chest, 154, 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH AK, GUPTA R, GHOSH A & MISRA A 2020. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 14, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISSON JH, WYATT TA, GUIDOT DM, BAGBY GJ, HELANDER A, TØNNESEN H & SPIES CD 2005. Bench to Bedside: Mechanisms and Consequences of Alcohol-Altered Host Defenses. Alcoholism: Clinical & Experimental Research, 29, 1090–1097. [Google Scholar]
- TENHOOR T, MANNINO DM & MOSS M 2001. Risk factors for ARDS in the United States: analysis of the 1993 National Mortality Followback Study. Chest, 119, 1179–1184. [DOI] [PubMed] [Google Scholar]
- TESTINO G 2020. Are patients with alcohol use disorders at increased risk for Covid-19 infection? Alcohol and Alcoholism, 55, 344–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOPALOGLU U & PALCHUK MB 2018. Using a federated network of real-world data to optimize clinical trials operations. JCO clinical cancer informatics, 2, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMNUAYPORNLERT A, KANCHANASURAKIT S, LUCERO-PRISNO DEI & SAOKAEW S 2021. Smoking and risk of negative outcomes among COVID-19 patients: A systematic review and meta-analysis. Tobacco induced diseases, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN WESTEN-LAGERWEIJ NA, MEIJER E, MEEUWSEN EG, CHAVANNES NH, WILLEMSEN MC & CROES EA 2021. Are smokers protected against SARS-CoV-2 infection (COVID-19)? The origins of the myth. npj Primary Care Respiratory Medicine, 31, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ZYL-SMIT RN, RICHARDS G & LEONE FT 2020. Tobacco smoking and COVID-19 infection. The Lancet Respiratory Medicine, 8, 664–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISWESWARAN S, BECICH MJ, D’ITRI VS, SENDRO ER, MACFADDEN D, ANDERSON NR, ALLEN KA, RANGANATHAN D, MURPHY SN & MORRATO EH 2018. Accrual to Clinical Trials (ACT): a clinical and translational science award consortium network. JAMIA open, 1, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG L, WANG Q, DAVIS PB, VOLKOW ND & XU R 2022. Increased risk for COVID‐19 breakthrough infection in fully vaccinated patients with substance use disorders in the United States between December 2020 and August 2021. World Psychiatry, 21, 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN KJ, POOLE JA, SWEETER JM, DEVASURE JM & WYATT TA 2019. An association between MMP-9 and impaired T cell migration in ethanol-fed BALB/c mice infected with respiratory syncytial virus-2A. Alcohol, 80, 25–32. [DOI] [PubMed] [Google Scholar]
- WARREN KJ, SIMET SM, PAVLIK JA, DEVASURE JM, SISSON JH, POOLE JA & WYATT TA 2016. RSV-specific anti-viral immunity is disrupted by chronic ethanol consumption. Alcohol, 55, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEINBERGER AH, PACEK LR, GIOVENCO D, GALEA S, ZVOLENSKY MJ, GBEDEMAH M & GOODWIN RD 2019. Cigarette use among individuals with alcohol use disorders in the United States, 2002 to 2016: trends overall and by race/ethnicity. Alcoholism: Clinical and Experimental Research, 43, 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU F, ZHOU Y, WANG Z, XIE M, SHI Z, TANG Z, LI X, LI X, LEI C, LI Y, NI Z, HU Y, LIU X, YIN W, CHENG L, YE F, PENG J, HUANG L, TIAN J, ZHANG L, MO X, ZHANG Y, HU K, JIANG Y, GUAN W, XIANG J, LIU Y, PENG Y, WEI L, HU Y, PENG P, WANG J, LIU J, HUANG W, CHEN R, ZHAO J, LI S, ZHANG N, ZHAO J, ZHONG N, RAN P, MEDICAL TREATMENT EXPERT GROUP FOR, C. & COVID 2020. Clinical characteristics of COVID-19 infection in chronic obstructive pulmonary disease: a multicenter, retrospective, observational study. Journal of thoracic disease, 12, 1811–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG J, ZHENG Y, GOU X, PU K, CHEN Z, GUO Q, JI R, WANG H, WANG Y & ZHOU Y 2020. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG H, MA S, HAN T, QU G, CHENG C, UY JP, SHAIKH MB, ZHOU Q, SONG EJ & SUN C 2021. Association of smoking history with severe and critical outcomes in COVID-19 patients: A systemic review and meta-analysis. European journal of integrative medicine, 43, 101313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHENG Z, PENG F, XU B, ZHAO J, LIU H, PENG J, LI Q, JIANG C, ZHOU Y & LIU S 2020. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. Journal of Infection [DOI] [PMC free article] [PubMed]
- ZUIN M, RIGATELLI G, ZULIANI G, RIGATELLI A, MAZZA A & RONCON L 2020. Arterial hypertension and risk of death in patients with COVID-19 infection: systematic review and meta-analysis. Journal of Infection, 81, e84–e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


