Figure 1.
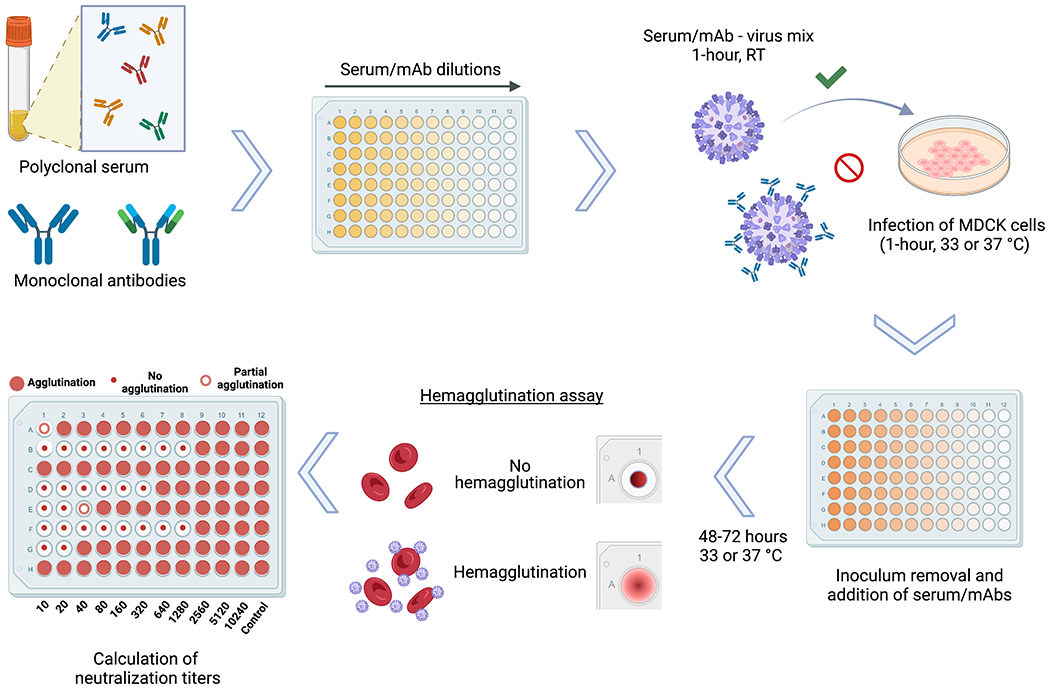
Schematic representation of the microneutralization assay for influenza virus serology. Antibodies contained in polyclonal serum samples of different origin or monoclonal antibodies (mAb) in distinct configurations including chimeric antibodies, Fab fragments and others, can be assessed for their neutralization capacity against different strains and subtypes of influenza viruses. After dilution of serum samples, the virus to be tested is added at 100TCID50/ml, followed by a 1-hour incubation period at room temperature (RT). Serum/mAb – virus mix is transferred to confluent MDCK cells and incubated for 1-hour at 33 or 37 °C depending on the virus strain used. After inoculum removal, serum samples diluted in infection media are added to the plates, followed by a 48-72 hour incubation at 33 or 37 °C depending on the virus strain used. A hemagglutination assay is performed to detect the presence of virus in the cell supernatants and calculate the neutralization titers.
