Figure 1. The general structure of ADAMs.
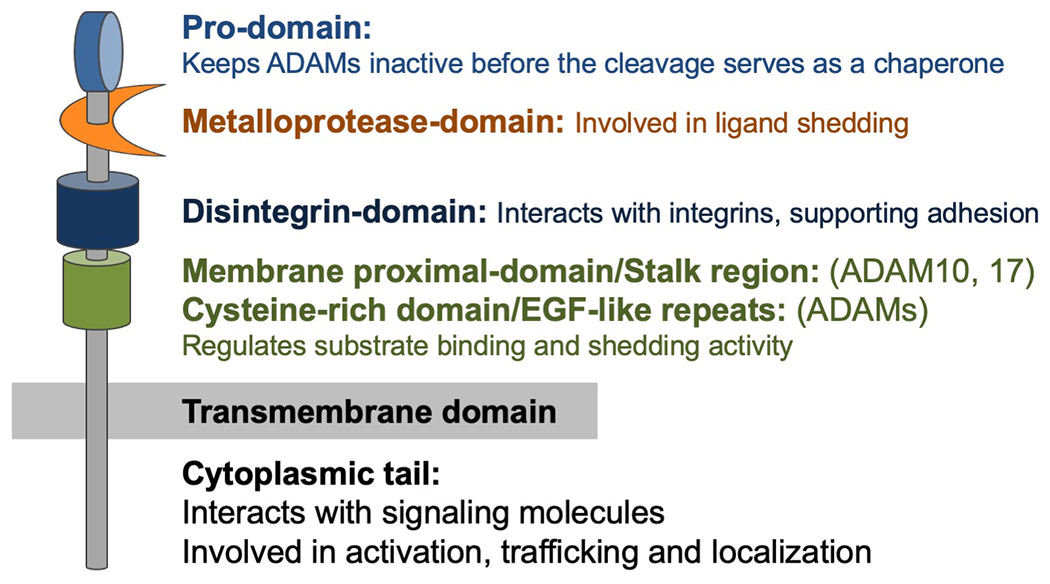
A disintegrin and metalloproteases (ADAMs) consist of several domains. The pro-domain keeps ADAMs inactive, and protein convertases such as furin cleave this pro-domain in Golgi apparatus to activate ADAM17. The metalloprotease-domain is a key domain that is involved in catalytic activity and ligand shedding. The disintegrin-domain interacts with integrins and supports adhesion. This domain also serves to maintain the structure of extracellular region. The membrane proximal domain regulates substrate binding and shedding activity. ADAM10 and ADAM17 have membrane proximal-domain and other ADAMs have EGF-like repeats, which regulate substrate binding and shedding activity. Cytoplasmic tail of ADAMs interacts with signaling molecules. Phosphorylation of cytoplasmic tail regulate the activation, trafficking and subcellular localization of ADAMs.
