Figure 4. Formation of MT ring via alternative dynein-kinesin balance shift mechanisms features cell rigidification as an outcome of MT network expansion.
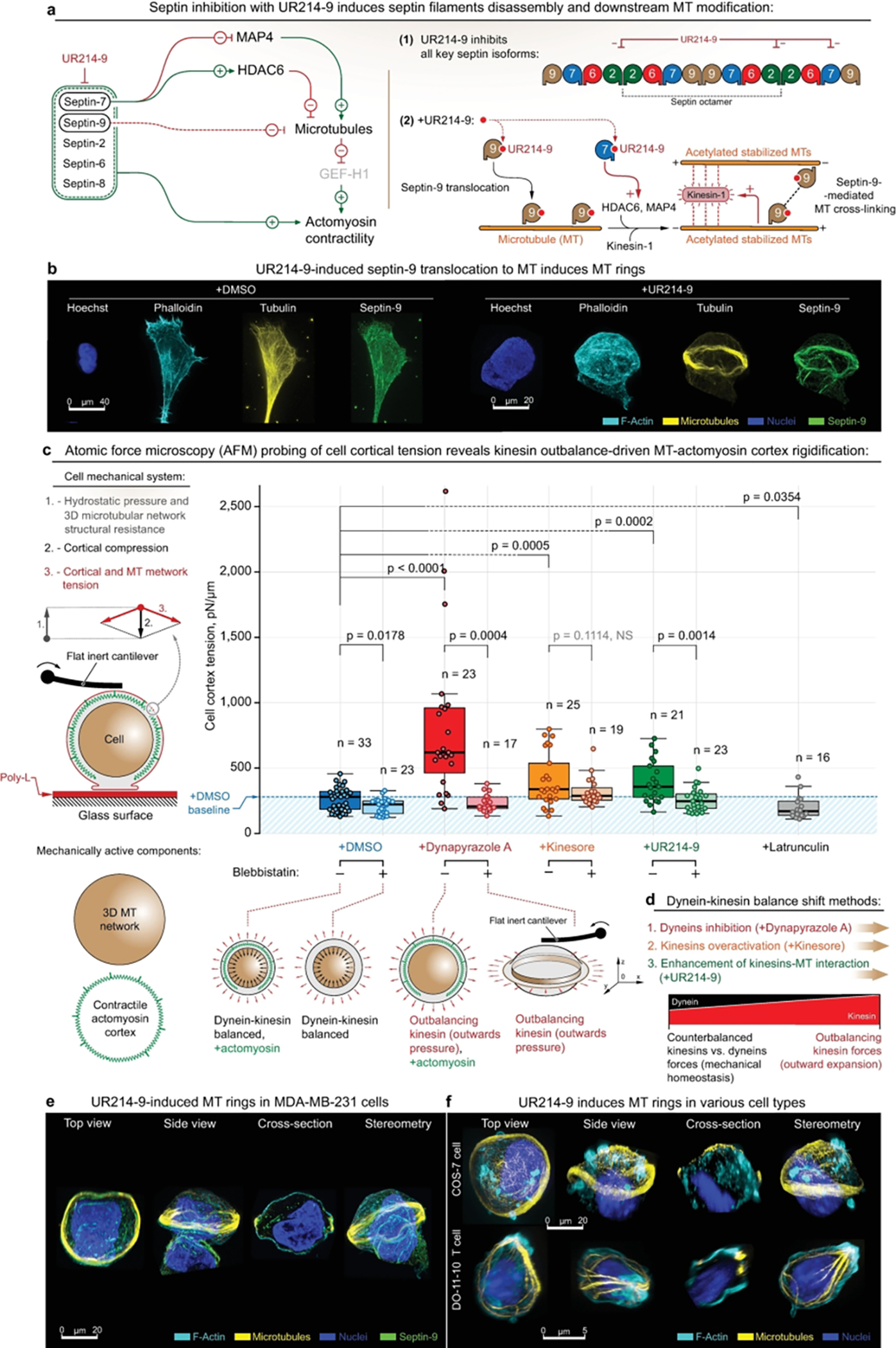
(a) - (Left) UR214–9-targeted septins blockade leads to the downstream stabilization of microtubules. I.e., septin-7 blockade leads to HDAC6 inactivation and following microtubules acetylation and stabilization, coherent with the parallel effects of MAP4 release from septin-7 to the microtubular pool. In addition, total septin suppression leads to the loss of actomyosin contractile structures (e.g. - stress-fibers), and to a total decrease of actomyosin contractility. (Right) Septin-9 blockade with UR214–9 leads to its relocation to the stabilized microtubules, where it increases microtubular cross-linking via direct septin-9 interactions, and possibly via F-actin mediation. Stabilized acetylated MT activates kinesin-1, while combined with MT cross-linking leads to the MT bundling and bundles extension.
(b) - Immunofluorescent visualization of stress-fiber-specific localization for septin-9 in MDA-MB-231 cells in control conditions (+DMSO), and septin-9 translocation onto MT network, accompanied by MT ring formation during septin-2 inactivation (+UR214–9). See also SI14a. For cells attached directly to the uncoated glass (for the purpose of super-resolution imaging), rings were formed in ~10–15% of total cell population, n=100 (estimates are made by visual examination). We attribute the quantitative differences with collagen gels to the nature of the substrates used in various experiments.
(c) - AFM analysis of the MDA-MB-231 cells rigidification upon kinesin-wise activity outbalance, induced by three alternative mechanisms. Left - Schematic of the cell mechanical system: both MT network and actomyosin represent mechanically active cell components that synergistically regulate cell mechanics. Right - Dynapyrazole A, Kinesore, and UR214–9 induce cell rigidification. Bottom - schematic representation of cell rigidification upon kinesin’s outbalancing activity: while +Dynapyrazole A+Blebbistatin, +Kinesin+Blebbistatin, and +UR214–9+Blebbistatin all induce MT rings, due to the rings’ anisotropic configuration their mechanical effects are non-readable by AFM.
(d) - Schematic representation of the alternative mechanisms for inducing the kinesin’s outbalancing activity. Both dynein inhibition (+Dynapyrazole A), kinesin over-activation (+Kinesore), and kinesin-MT interactions enhancement via septin-9 F-actin-to-MT translocation (+UR214–9) unanimously induce cell rigidification via kinesin-driven MT network expansion. See also SI9b.
(e, f) - UR214–9-induced kinesin overactivation results in MT rings formation in MDA-MB-231 cells (e), as well as in other tested cell lines, e.g. - COS-7 (f, top, rings were formed in ~10–20% of total cell population, n=100, estimates are made by visual examination), and in DO-11–10 murine T cell line (f, bottom, rings were formed in ~8–15% of total cell population, n=100, estimates are made by visual examination).
F-actin is labelled with Phalloidin-ATTO 647N. Chromatin is labelled with Hoechst. Pairwise one-way t tests-derived p values are shown on the plots with corresponding n (size of individual cells measurement sets) for each condition, generated in triplicates.
