Figure 2. Screening anti-FGFR4 CAR T cells for specificity and function.
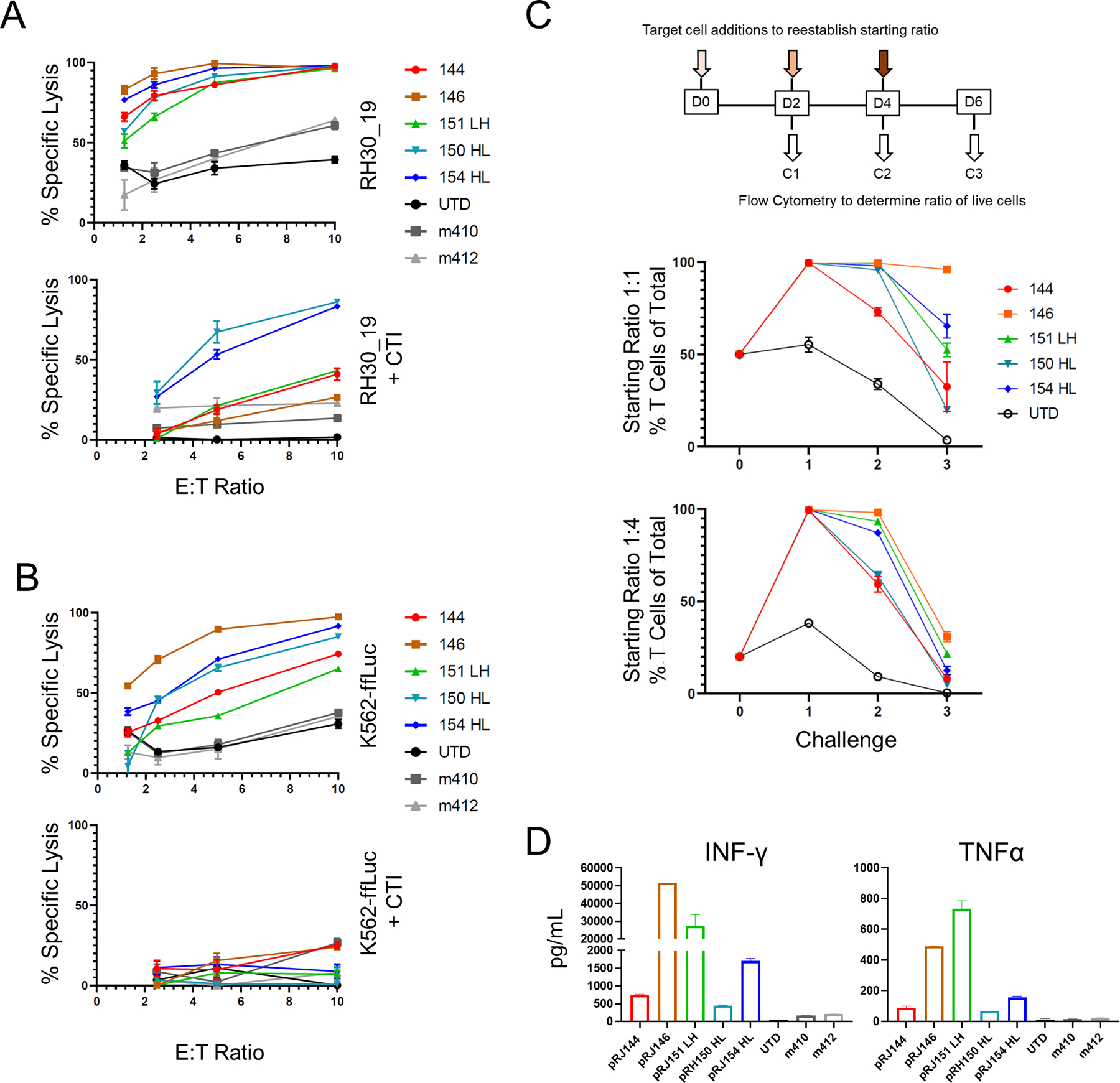
A, B Top candidate FGFR4 binders were tested for cytotoxicity against RH30_19 (A) or K562-ffLuc (B) with or without cold target inhibition (+CTI) using unlabeled K562 cells at 30-fold excess. C. Multiple challenge cytotoxicity assay was conducted by plating anti-FGFR4 CAR T cells with RH30_19 or Raji cells at a starting E:T ratio of 1:1 or 1:4. Cell populations were assessed by counting and flow cytometry at 48 hours, then original ratios re-established. This was repeated for three tumor-cell challenge cycles and the percentage effector T cells of the total population graphed at the end of each cycle (%T cells of total). Thus, 100% (y-axis) indicates no tumor detectable. D. Cytokine production at 20 hours of co-culture with RH30_19 target cells. All assays were conducted in triplicate and independently repeated at least twice.
