Abstract
Objective:
To summarize the pharmacology of the calcitonin peptide family of receptors and explore their relationship to migraine and current migraine therapies.
Background:
Therapeutics which dampen calcitonin gene-related peptide (CGRP) signaling are now in clinical use to prevent or treat migraine. However, CGRP belongs to a broader peptide family, including the peptides amylin and adrenomedullin. Receptors for this family are complex, displaying overlapping pharmacological profiles. Despite the focus on CGRP and the CGRP receptor in migraine research, recent evidence implicates related peptides and receptors in migraine.
Methods:
This narrative review summarizes literature encompassing the current pharmacological understanding of the calcitonin peptide family, and the evidence which links specific members of this family to migraine and migraine-like behaviors.
Results:
Recent work links amylin and adrenomedullin to migraine-like behavior in rodent models and migraine-like attacks in migraineurs. We collate novel information that suggests females may be more sensitive to amylin and CGRP in the context of migraine-like behaviors. We report that drugs designed to antagonize the canonical CGRP receptor also antagonize a second CGRP-responsive receptor and speculate as to whether this influences therapeutic efficacy. We also discuss the specificity of current drugs with regards to CGRP isoforms and how this may influence therapeutic profiles. Lastly, we emphasize that receptors related to, but distinct from, the canonical CGRP receptor may represent underappreciated and novel drug targets.
Conclusion:
Multiple peptides within the calcitonin family have been linked to migraine. The current focus on CGRP and its canonical receptor may be obscuring pathways to further therapeutics. Drug discovery schemes which take a wider view of the receptor family may lead to the development of new anti-migraine drugs with favorable clinical profiles. We also propose that understanding these related peptides and receptors may improve our interpretation regarding the mechanism of action of current drugs.
Keywords: CGRP, adrenomedullin, amylin, migraine, calcitonin receptor, receptor activity-modifying protein
Introduction
Migraine and the calcitonin family of peptides
Migraine is a common and debilitating neurological condition, with attacks being characterized by symptoms such as visual aura, sensitivity to light, nausea, and moderate to severe headache. This range of symptoms implicates many biological systems in the underlying mechanisms of migraine. Migraine is the leading cause of disability-adjusted life years in females aged 15–49, and is the second highest cause of disability-adjusted life years in the general population.1 Drugs designed to reduce the signaling of calcitonin gene-related peptide (CGRP) are now in clinical use for the prevention and treatment of migraine attacks. These drugs work by targeting either the CGRP peptide (galcanezumab, fremanezumab, eptinezumab), or CGRP-responsive receptors (erenumab, atogepant, ubrogepant, rimegepant). These drugs have been transformative for some patients, however ~40% of patients do not respond to these treatments, indicating that there is substantial room to improve on the current therapeutic landscape.2
CGRP belongs to a peptide family comprising calcitonin, amylin, adrenomedullin (AM), and adrenomedullin 2/intermedin (henceforth referred to as AM2). Additionally, CGRP itself exists in two forms, αCGRP and βCGRP (sometimes also known as CGRP-I and CGRP-II, respectively). Although the overall amino acid sequence identity within the family is low (Figure 1), all peptides share several features such as amidated C-termini, and a loop structure in the N-terminus created by a disulfide bond between the two conserved cysteine residues.3
Figure 1.
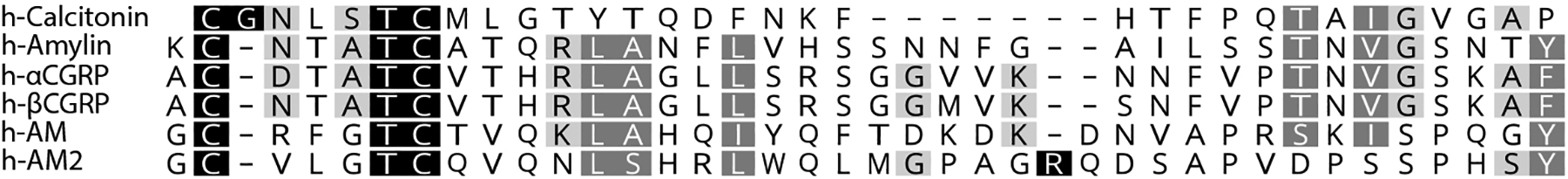
Amino acid sequence alignment of human peptides. Alignment performed using Clustal. Black background with white text indicates an exact match across peptides at a given position, dark grey with white text indicates >80% similarity across a position, and light grey with black text indicates 60–80% similarity across a position; scoring was performed in Geneious using a BloSUM matrix of 80 and a threshold of 2. The AM and AM2 sequences shown here omit the N-terminal 14 and 13 amino acids of each peptide, respectively. These N-terminal amino acids do not appear to be required for peptide function3 and are thus omitted from the figure, however they were included in the original alignment.
These peptides have diverse physiological functions; although CGRP is commonly associated with migraine it also plays roles in cardioprotection and regulation of gastrointestinal functions.4 AM and AM2 have protective roles in the cardiovasculature, while also influencing reproductive and renal function.5–7 Amylin is a metabolic hormone which reduces gastric emptying and regulates satiety.8, 9 Pramlintide, an amylin-mimetic, is used therapeutically in the management of diabetes; novel amylin-mimetics are also being explored for managing bodyweight and appetite.10 Calcitonin stimulates bone growth; salmon calcitonin is used clinically for the treatment of osteoporosis.11Calcitonin analogues are also being developed for the management of obesity and diabetes.12 CGRP is the most prominent member of this family currently linked to migraine, but growing evidence suggests that other peptides within the family could also play a role. In this review we collate pharmacological data regarding this peptide family and summarize evidence linking these peptides to migraine.
Methods
We performed a narrative review of literature using PubMed, Google Scholar and Scopus. The final searches were performed on the 28th of April 2022. Descriptions of receptors and peptides were derived from the International Union of Basic and Clinical Pharmacology maintained “Guide to Pharmacology” database (available at https://www.guidetopharmacology.org/); this was supplemented by searching PubMed with search terms including “pharmacology” combined with “CGRP”, “adrenomedullin”, or “amylin”. Results from in vivo studies using these peptides were also collated for this review. The search terms included “CGRP”, “amylin”, or “adrenomedullin” in combination with “pain”, “migraine”, “light aversion”, or “hyperalgesia”. Article titles and full texts were screened to determine relevance for inclusion. In addition, reference lists of these articles were examined to identify further studies for inclusion. We have focused on primary data sources in our citations, however in certain sections we have also cited reviews to draw these to the reader’s attention.
Results
Pharmacology of the calcitonin family of receptors
Receptors for the calcitonin peptide family consist of two class B G protein-coupled receptors (GPCRs); the calcitonin receptor (CTR), and the calcitonin receptor-like receptor (CLR), both of which can interact with accessory proteins known as receptor activity-modifying proteins (RAMPs).3, 13 These protein-protein interactions give rise to receptors that each have a distinct recognition profile for different combinations of calcitonin family peptides. Thus, the interaction between CLR and RAMP1 creates the canonical CGRP receptor, which has high affinity for αCGRP and βCGRP.3, 14 This is the receptor that drug discovery programs leading to the development of receptor-targeting drugs, such as erenumab and the “gepants”, have been targeted towards. Complexes comprising CLR and RAMP2 or RAMP3 create the AM1 or AM2 receptors, respectively; these receptors have high affinity for AM and AM2.3, 15 The CTR can also complex with RAMPs; these interactions give rise to the AMY1 (CTR:RAMP1), AMY2 (CTR:RAMP2), and AMY3 (CTR:RAMP3) receptors.13 These receptors all display a high affinity for amylin, however the AMY1 receptor also displays high affinity for CGRP.3, 16–18 CLR is unable to translocate to the cell surface or act as a peptide-ligand receptor on its own, this contrasts with CTR which can traffic to the cell surface and act as a receptor for calcitonin in the absence of RAMPs.3 The ability of CTR to translocate to the cell-surface alone means that any individual cell which expresses CTR and RAMPs could theoretically express a mixed population of CTR and AMY receptors on the cell surface. It is unclear whether this has physiological implications in vivo, however it does complicate the interpretation of in vitro work where it is likely that cells transfected with CTR and a RAMP express a combination of CTR and AMY receptors at the cell surface.3 This has previously lead to the over-estimation of the potency of human calcitonin at AMY receptors.3, 16The nomenclature suggests that each of these receptors has clear-cut pharmacology, however there is actually substantial overlap in the ligand preference of each receptor.3 This is illustrated in Figure 2 by showing that several human, rat, and mouse receptors are activated by CGRP.3, 19–21 This overlapping pharmacology means that each receptor could potentially function as a receptor for multiple distinct endogenous peptide ligands in vivo. This complex pharmacology also makes it difficult to directly link a given receptor complex to defined (patho)physiological scenarios because administration of a single peptide can theoretically activate multiple receptors from this family. This problem is exacerbated in rodents, where each peptide tends to activate more individual receptors, or activate them more potently than human receptors (Figure 2). This means that peptide administration to humans or animals could activate a wide range of receptors. For example, in mice αCGRP has nanomolar potency at four of the seven receptors from this family.19 This causes serious difficulties for the interpretation of in vivo results. Efforts are being made to probe the relative importance of individual receptors in different physiological pathways using a combination of pharmacological and genetic approaches.22–25 More work like this is needed.
Figure 2.
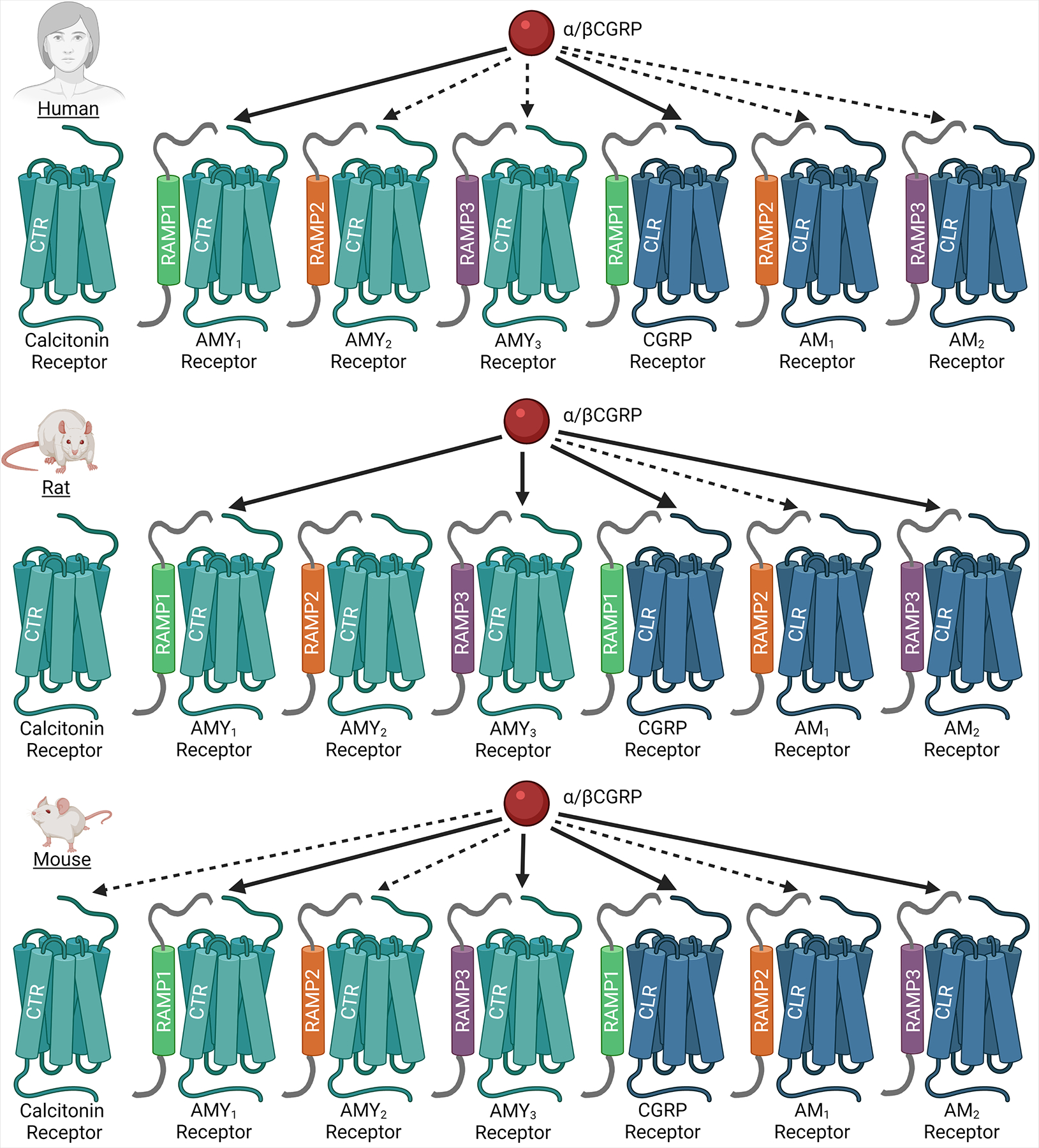
Pharmacology of CGRP at human, rat, and mouse CTR/CLR-based receptors. Undashed arrows indicate receptor at which CGRP is either the most potent peptide, or where CGRP is less than 10-fold weaker than the most potent peptide. Dashed arrows indicate receptors at which CGRP is between ~10-fold and 100-fold weaker than the most potent peptide. Visualizations are approximate, please see references for a more in-depth discussion of results3, 19, 21. Image created using BioRender.com.
Migraine-provocation studies in which humans have been administered calcitonin family peptides have offered great insight into the molecular mechanisms of migraine, however they have also raised a number of questions. For instance, peptides such as CGRP and adrenomedullin are rapidly metabolized by proteases and peptidases in blood,5, 26, 27 so their long-lasting effects are difficult to explain. Additionally, intravenous administration delivers peptides to the peripheral blood supply. The blood-brain barrier excludes molecules of similar size and charge to CGRP, amylin, and adrenomedullin,28–30 and the penetrance from peripheral blood to central sites is very low for these peptides.28, 31–33 This means that it is unlikely that these peptides are reaching intracranial receptors and instead suggests that migraine-like attacks induced by these peptides given peripherally could be driven by activation of peripheral receptors. The trigeminovascular system is a conduit for orofacial pain and is thus intimately linked to migraine pathophysiology.35, 36 Importantly, the trigeminovascular system also receives blood from the peripheral supply,28, 30 leading to the idea that migraine-inducing agents such as CGRP act in the trigeminovascular system to cause migraine attacks.28, 34, 37, 38 The importance of these peripheral receptors is supported by the fact that antibody therapeutics have limited central penetrance,39–41 but still have therapeutic efficacy in patients. More work investigating the relative importance of peripheral and central sites in migraine pathophysiology is needed.
αCGRP and βCGRP in migraine
There is a wealth of literature linking CGRP to migraine pathophysiology in humans and rodents, the findings of which have recently been reviewed.34, 35, 37, 42–44 These reviews effectively cover a lot background, and it is not our intention to replicate them. This section will instead introduce methods and themes used in migraine research and highlight new insights in the field.
We will first consider pre-clinical rodent models. Rodents are often used to probe mechanisms of disease in a way that is not feasible in humans. Various rodent models have therefore been developed to interrogate the different facets of the human migraine experience. Facial signs of discomfort (which can be measured through grimace and squint scales) capture the spontaneous symptoms of pain and discomfort arising during migraine attacks such as headache and nausea; CGRP administration elicits both grimace and squint responses.45–48 Mechanical and thermal assays which measure hyperalgesia and allodynia recapitulate the hypersensitivity commonly associated with migraine attacks; exogenous CGRP administration reduces pain thresholds in both mechanical and thermal assays.49–52 Light aversive behaviors, in which rodents spend longer times resting in the dark and avoid illuminated spaces, recapitulate the hypersensitivity to light which is commonly associated with a migraine-attack; CGRP administration to rodents elicits light aversive behaviors.48, 53, 54 Collectively, these animal studies provide weight to the importance of CGRP in migraine pathophysiology.
Recent rodent work has also indicated that there may be a sex-specific element to CGRP action. For example, dural application of CGRP causes periorbital allodynia in female but not male rodents.47, 55 Intraperitoneal injections of CGRP cause light aversion and squint responses in both male and female mice,45, 48, 56 though recent evidence suggests that these squint responses occur at lower doses in females.45 These findings may be especially relevant given that migraine has a higher prevalence in women.1 CGRP also appears to regulate other pain responses more potently in females than males, for example interleukin-6 primed mechanical hyperalgesia.57 Thus, CGRP may also have a broader role in pain processing in a sex-dependent manner. This is an avenue worth investigating and future studies may extend the therapeutic repertoire of CGRP receptor antagonists beyond migraine to other chronic pain conditions.57
The clinical evidence implicating CGRP in the human migraine response has been extensively reviewed and thus will not be developed in detail in this review.37, 42, 58–60 To summarize key points from this work: CGRP and its receptors are expressed in the trigeminovascular system,35, 43, 61–65 CGRP levels are increased in circulating bodily fluids during migraine-attacks,66, 67 though this has finding has not always been reproducible;68 intravenous infusion of CGRP to migraineurs provokes migraine-like attacks;69 and blockade of CGRP activity can reduce the incidence of migraine attacks in migraineurs.2, 70–73 As a consequence of such observations and much other research, we are now in a position to have multiple therapeutics available that target either the CGRP peptide or receptor to reduce CGRP-mediated signaling. The efficacy of these drugs has further strengthened the evidence-base for CGRP being a key contributor to migraine symptoms. Although effective in many patients, they are not effective in all,71–77 and some patients experience side effects, such as constipation.76, 77
Genome-wide association studies (GWAS) are starting to provide some insights. In one study, a novel migraine-associated risk locus included the genes for both αCGRP (CALCA) and βCGRP (CALCB), however the resolution of this study was not sufficient to distinguish between the two genes.78 A second study, which was more targeted than the previous study, identified a strong link between the CALCB locus and migraine.79 Additionally, a retrospective literature analysis identified a link between the CALCA locus and migraine though the clinical significance of this was unclear;80 this study also identified a variant in the RAMP1 locus that is associated with the progression from episodic migraine to medication overuse headache and noted a potential link between the methylation state of the RAMP1 promoter region and migraine.80, 81 Thus, it appears that there may be subtle genetic susceptibilities in the CGRP-CGRP receptor pathway that could predispose individuals to migraine or affect the behavior of anti-CGRP therapeutics.
CGRP is most commonly associated with the canonical CGRP receptor (CLR:RAMP1) but CGRP can also bind to and activate the AMY1 receptor (CTR:RAMP1) with high affinity and potency (Figure 2).3, 17, 18 The canonical CGRP receptor is often presumed to be the predominant CGRP-responsive receptor, however, CTR mRNA and protein expression is noted in the trigeminal ganglia in addition to CLR and RAMP1,20, 62, 65, 82 and CTR is found in other migraine-relevant locations of the brain such as the locus coeruleus and nucleus raphe magnus.83, 84 Thus, both CGRP-responsive receptors could play a role in migraine. It is prudent to add that some studies note low expression of CTR mRNA in sites such as the trigeminal ganglia,82 but are able to detect CTR protein in the same tissue through immunohistochemical techniques.20, 82 This apparent contradiction could be explained by the fact that low levels of mRNA can still lead to protein production.85, 86 It could also be the result of mRNA being synthesized and translated in neuronal cell bodies, with the protein then being trafficked to a remote fiber, leading to protein expression at sites distant to the original mRNA synthesis.85, 86
Adding weight to the idea that both CGRP-responsive receptors could be important to migraine is the mounting evidence showing that drugs designed to target the canonical CGRP receptor also have antagonist activity at the AMY1 receptor.20, 87–90 The effects of these drugs could therefore be due in part to their activity at AMY1 receptors. The molecular mechanism of this dual antagonism can be interpreted through crystal structures which have captured complexes of gepants and erenumab bound to the extracellular domains of CLR:RAMP1 [PDB 3N7R and PDB 6UMG, respectively].91, 92 Both drug types bind to the extracellular domain of the CGRP receptor, making contacts with both CLR and RAMP1. It is thought that this occludes the CGRP binding pocket to prevent the ligand-receptor interaction.91, 92 Given that RAMP1 is shared between the CGRP and AMY1 receptors, and that the extracellular domains of CLR and CTR have a similarity score of >70% (BLOSUM 75 matrix with threshold 0), it is perhaps not surprising that these drugs display some effect at the AMY1 receptor.87–90 Future studies investigating whether CGRP induces migraine-like symptoms via individual receptors or through a combination will be important for understanding and more effectively treating migraine.
Finally, it is worth noting that work in this space has generally focused on αCGRP. It is not known whether αCGRP and βCGRP have differential effects in migraine. Given that αCGRP and βCGRP are both expressed in the trigeminal ganglia,93–95 are both identified in GWAS studies as linked to migraine,78–80 and exhibit overlap in binding sites across the nervous system,96, 97 it is likely that βCGRP plays some role in migraine. Interestingly, βCGRP is often reported to be a little more potent than αCGRP in vitro,3, 98–100 has a higher affinity than αCGRP in the rat central amygdala,97 and has a higher density of binding sites than αCGRP in the ventromedial hypothalamus,96 therefore future studies should specifically investigate this peptide and its link to migraine.
Amylin and migraine
Amylin is a 37 amino acid hormone best known for its roles in glucoregulation and meal-ending satiation.8, 101 Amylin is expressed in islet β-cells in the pancreas and is released alongside insulin in response to glucose.8, 101 Amylin expression has, however, also been reported in a few other locations. Notably, its apparent expression in the dorsal root ganglia suggests a role for amylin in nociception.102 A few studies have also reported amylin-like immunoreactivity in neurons of the trigeminal ganglia.56, 82, 103 However, when CGRP and amylin immunoreactivity in normal trigeminal ganglia are directly compared, there are two important observations. Firstly, the amylin-like immunoreactivity is much weaker, as compared to CGRP-like immunoreactivity,56, 82 and secondly, the amylin-like immunoreactivity that is present strongly overlaps with CGRP;56, 82 both points are made clear when comparing amylin and CGRP expression in a single section of tissue.56 An explanation for this is that the “amylin” staining reported in the trigeminal ganglia most likely represents “off-target” detection of CGRP, which is expressed at high concentrations in densely packed vesicles in these neurons,104 as opposed to actual amylin expression. This could occur because many “amylin-targeting” antibodies which have been used to detect amylin in the trigeminal ganglia also recognize CGRP,105 which is perhaps not surprising given the high sequence similarities between the two (Figure 1). In light of this, further work is needed to carefully scrutinize the potential expression of amylin outside of the pancreas, where mRNA for amylin is compared to amylin protein expression, and expression of amylin is directly compared to CGRP with well-validated antibodies.
Regardless of the ambiguity around its endogenous site(s) of expression, amylin has been functionally linked to pro-nociceptive and migraine-like behaviors in rodents. It is worth noting that amylin is a relatively potent agonist of several receptors in mice, including the mouse AM2 receptor, as well as AMY receptors.19 Therefore, any functional responses noted in mice could potentially be mediated by multiple receptors. Mice lacking amylin display reduced nociceptive behavior in response to intraplantar formalin injection.102 In female mice, intraperitoneal administration of amylin decreases mechanical pain thresholds, reduces time spent in the light, and induces squint responses.45, 56 Interestingly, in male mice intraperitoneal amylin administration did not cause squint responses or light-aversive behaviors, however it did cause a reduction in plantar mechanical pain thresholds.56 A recent study reported that subcutaneous amylin did not induce periorbital allodynia in male mice.52 Given that migraine skews heavily female in human populations,1 and that female mice appear to be more sensitive to amylin,45, 56 negative results obtained from male-only studies should be interpreted with caution. This also emphasizes the importance of including female rodents in preclinical studies, especially when studying a phenomenon such as migraine which is more common in women. In contrast to the above nociceptive actions of amylin, other studies report anti-nociceptive effects of amylin; intraperitoneal administration of amylin reduces acetic acid-induced visceral pain while intracerebroventricular injection reduces thermal nociception.106, 107 Thus, the effects of amylin may be dependent on sex, route of administration or nociceptive pathways measured. Another factor to consider is pharmacokinetics. When administering amylin via peripheral injection, it reaches a maximum plasma concentration within ~10 minutes and is rapidly cleared.108 Interestingly, amylin causes a squint response at earlier time-points than CGRP.45 Hence, in an acute study setting, selecting the correct time points is crucial. Additionally, amylin appears to require higher doses to elicit the same pain responses as CGRP,45, 56 and this should be factored into study design.
Despite mixed results in rodents, a recent human study reported that the amylin mimetic, pramlintide, induced migraine-like attacks in migraineurs. Using a double-blind crossover approach, migraineurs were administered CGRP (30 μg over 20 minutes) or pramlintide (120 μg over 20 minutes) and headache scores monitored. Pramlintide induced headache and migraine-like attacks in 88% and 41% of patients, respectively; this was not significantly different to CGRP, which induced headache and migraine-like attacks in 97% and 56% of patients, respectively.56 Despite the rate of headache and migraine-like attacks being similar between peptides, CGRP-induced symptoms were significantly more painful than pramlintide-induced symptoms.56 In all cases the migraine-like attacks mimicked the patients’ usual migraine symptoms.56 Interestingly, there was a subset of patients who responded only to CGRP, but also a subset of patients who responded only to pramlintide56 suggesting that some migraineurs could be hypersensitive to amylin in addition to, or instead of, CGRP. Given that pramlintide has only weak activity at the human CGRP receptor,56, 109 it suggests that activation of AMY receptors is sufficient for induction of migraine-like attacks in some patients. When combined with other data showing that plasma amylin levels appear to be elevated in chronic migraineurs during the interictal phase of migraine,110 it is possible that endogenous amylin plays a role in migraine. FDA documents for pramlintide report that headache is a relatively common side-effect of pramlintide use,111 however the studies leading to this listing were not designed to measure headache or migraine intensity. Further studies with larger sample sizes which investigate pramlintide as a migraine-inducing agent, and studies that investigate the relative contribution of each AMY receptor in the migraine response will be valuable. It is possible that AMY2 and AMY3 receptors play a role in migraine, as both RAMP2 and RAMP3 are expressed in the trigeminal ganglia,34, 82 however at this time the role of each receptor remains an open question. It is very challenging to separate the roles of the different AMY receptors in human studies because we currently lack pharmacological probes specific enough to distinguish individual AMY receptors. This means that we will likely need to rely, for now, on genetic manipulation of specific receptor components in rodents to link defined receptors to specific functional effects.
AM and migraine
AM is expressed at high concentrations by the vascular endothelium.5, 82, 112, 113 AM is a powerful vasodilator, playing important roles in vascular maintenance and regulation of the blood-brain barrier.5, 112–114 At human receptors, AM is most potent at AM1 and AM2 receptors, however it also has activity at the CGRP receptor, being between 10-fold and 100-fold less potent than αCGRP itself.3, 14, 99 Although not commonly associated with migraine, AM expression is noted in neurons of the dorsal root ganglia hinting at a role in pain processing pathways.115 Within the trigeminal ganglia, AM expression has been reported in glial and Schwann cells.82
Consistent with other calcitonin family peptides, AM has relatively potent activity at more receptors in mice, than in humans. For example, the mouse AMY3 receptor stands out as potentially mediating AM effects in mice.19 Rodent studies investigating AM in nociception have been dependent on the pain pathway measured. AM knockout mice have increased tail-flick latency in response to heat; however, they also have decreased paw withdrawal latency in response to heat.116 Intrathecal administration of AM induces mechanical hyperalgesia and inflammatory pain in the paw.117, 118 There has been no investigation into AM induced non-nociceptive migraine-like responses such as light aversion. Administration of AM to male mice did not induce periorbital allodynia;52 the equivalent experiments have not been performed using female mice.
The ability of AM to induce migraine-like attacks in humans has been explored with mixed results. The first investigation was a double-blinded placebo-controlled two-way crossover study which reported results from 12 participants; the AM dosage used in this study was 1.6 μg/kg.119 In this initial study 33.3% (4/12) of patients reported migraine-like attacks after AM administration; this was identical to the number of patients who reported migraine-like attacks after placebo administration.119 More patients reported immediate and delayed headaches after AM (50% [6/12] and 58.3% [7/12] of patients, respectively) than placebo (8.3% [1/12] and 33.3% [4/12] of patients, respectively) though this difference did not reach statistical significance.119 It is worth noting that the nocebo migraine response rate in this study (33.3%) is higher than the average nocebo response rate in migraine provocation studies (95% confidence intervals of 2.5% – 15.5%)120 and it is possible that this high nocebo rate may have confounded interpretation of results. Recently, a second double-blinded placebo-controlled two-way crossover study reported results from 20 patients; this study dosed AM at 2.4 μg/kg.121 In this study 55% (11/20) of patients reported migraine-like attacks after AM infusion, which was significantly higher than the 15% (3/20) of patients who reported migraine-like attacks after placebo.121 Unlike the previous study, headache analysis was not separated into immediate and delayed phase, however similar to the previous study more patients reported headache after AM infusion than after placebo infusion (80% [16/20] and 55% [11/20] of patients, respectively).121 When considering migraine-like attacks in the second study, the time to onset and distribution of pain around the head was similar between AM-induced and CGRP-induced migraine-like attacks.121 Additionally, AM was associated with headache induction in other human studies designed to explore the vascular effects of AM.122, 123 Thus, it appears that at high concentrations AM can induce headache and migraine-like attacks, however whether this could occur at physiologically relevant concentrations is unclear. It is also unknown which AM-responsive receptor/s are responsible for this migraine-like response, as the estimated blood concentrations reached in these studies would likely be able to at least partially activate the CGRP receptor as well as the AM1 and AM2 receptors.121 To differentiate the involvement of specific receptors in humans, a gepant could be used to determine whether this was able to block AM-induced migraine-like headache. This experiment would be useful because at human receptors AM strongly favors CLR-based receptors, and gepants have very low affinity for AM receptors.124–126 Thus, if blocked by a gepant, it would be reasonable to conclude that the canonical CGRP receptor was mediating the response to AM.
Migraine therapeutics in the context of the wider calcitonin family of peptides and receptors
Despite the undoubted success associated with developing drugs that modulate CGRP signaling, there are a number of questions that arise when considering targets beyond the canonical αCGRP-CLR:RAMP1 axis. For instance, the three CGRP-targeting drugs (eptinezumab, galcanezumab, fremanezumab) appear to bind αCGRP and βCGRP non-specifically.127–129 The clinical relevance of this is unclear. αCGRP and βCGRP display different relative expression patterns in the body, with αCGRP being more highly expressed in the central nervous system and βCGRP being more highly expressed in the enteric nervous system however, there is overlap in expression with βCGRP also being found in migraine-related tissues such as the trigeminal ganglia.93, 94, 130 It is not known whether endogenous βCGRP plays a role in migraine, however given that the gene encoding βCGRP has been identified as potentially linked to migraine in GWAS work it is plausible.79 It could be that the ability of these antibodies to recognize both CGRP isoforms is important for their clinical efficacy, alternatively, the inability of these antibodies to differentiate the two isoforms could be responsible for side-effects such as the constipation reported in ~20% of patients taking anti-CGRP antibodies.77
There are also important unanswered questions regarding the receptors which mediate the effects of CGRP in vivo. Although receptor-targeting drugs are often considered specific for the canonical CGRP receptor, there is clear ability of many of these drugs to also antagonize the AMY1 receptor.87–90 The “gepants” display an ~30–100-fold preference for the CGRP receptor over the AMY1 receptor through the canonical cAMP signaling pathway;87–89 this preference can be as low as 10-fold in other pathways.88 This effect is not limited to small molecules as erenumab has been shown to antagonize both CGRP at the CGRP receptor and amylin at the AMY1 receptor,90 although the key experiment of erenumab antagonizing CGRP at the AMY1 receptor has not been performed. This precludes a direct comparison of erenumab activity at both receptors, however it suggests that erenumab can act at both CGRP-responsive receptors. The monoclonal antibodies which target the CGRP peptide by default reduce CGRP activity at both the CGRP and AMY1 receptors. Thus, the pharmacological effects of either class of drugs could be a result of their direct or indirect influence on CGRP and AMY1 receptor activity. The relative contribution each receptor makes to the overall migraine response is unclear, meaning that we do not know whether this dual-antagonism is beneficial for migraine therapies, whether we could reduce side-effects by targeting only one of these receptors, or whether this dual antagonism is incidental and irrelevant to the drug profile. Future studies which disentangle the role of each receptor in migraine will be invaluable to understanding the mechanism of action for these drugs.
An emerging phenomenon is co-administration of multiple classes of anti-CGRP pathway drugs.131–134 Preliminary clinical reports show that these combinations can be safe and more effective than each approach individually.131–134 Interestingly, the anti-migraine effects appear additive when gepants are combined with either peptide-targeting antibodies (such as galcanezumab),131, 132 or receptor-targeting antibodies (erenumab).131, 133 The former finding is somewhat intuitive; blockade of both parts of the CGRP:CGRP/AMY1 receptor signaling pathway is likely to be more effective at reducing CGRP signaling than targeting a single component of the pathway. The latter finding is perhaps more interesting and suggests erenumab does not block 100% of the CGRP-responsive receptors in the body on its own. This could be explained to circulating concentrations of erenumab not being high enough to target all migraine-relevant receptors, or due the gepants and erenumab having different distributions across the body. It is also possible that that this additive effect is due to the gepants and erenumab having different relative abilities to antagonize the CGRP and AMY1 receptors; full pharmacological profiles of these compounds will help us understand whether this is a viable explanation.
Conclusion
Drugs targeting the CGRP system are success stories in the development of migraine therapeutics, however there is still significant scope to improve upon these drugs to reach better clinical endpoints in larger proportions of the patient population. The focus on CGRP and CGRP receptor has led to the success we have today; however, there is a growing body of evidence to suggest that receptors outside the canonical CGRP receptor play a role in migraine (Table 1). Taking a holistic view of this receptor/peptide family may unlock additional targets, leading to improvements in current therapies.
Table 1.
An overview of calcitonin-family peptides and their functional links to migraine. The table is divided into results from studies investigating mice (m) rats (r) or humans (h). Receptors in bold are those at which the named peptide is either the most potent peptide, or has a potency within 10-fold of the most potent peptide, while receptors in italics are those at which the peptide has a potency between 10-fold and 100-fold lower than the most potent agonist (data are approximate only, please see references3, 19, 21, 136 for deeper discussion). Migraine-like behaviors in mouse and rats include squint responses, light aversion, and hyperalgesia/allodynia (mechanical and thermal). Y – statistically significant induction of the symptom in response to peptide infusion, N – no statistically significant induction of symptoms.
| Peptide | Receptors activated (m) | Migraine-like behavior (m) | Receptors activated (r) | Migraine-like behavior (r) | Receptors activated (h) | Headache (h) | Migraine-like attack (h) |
|---|---|---|---|---|---|---|---|
| CGRP | CGRP, AMY1, AMY2, AMY3, AM2, CTR, AM1 | Y44, 45, 47, 52, 54–56 | CGRP, AMY1, AMY3, AM2, AM1 | Y44, 47, 55, 57 | CGRP, AMY1, AMY3, AM1, AM2 | Y36, 37 | Y35, 37 |
| Amylin/Pramlintide | AMY1, AMY2, AMY3, AM2, CTR | Y45, 56, 102/N52, 106 | AMY1, AMY3, AMY2 CTR | Y135/N107 | AMY1, AMY2, AMY3, CTR | Y56 | Y56 |
| AM | AM1, AM2, AMY3, AMY2, CGRP | Y116/N52, 116 | AM1, AM2, CGRP, AMY1, AMY3 | Y115, 117, 118 | AM1, AM2, CGRP | Y121–123/N119 | Y121/N119 |
Funding:
MLG acknowledges receipt of a New Zealand Neurological Foundation First Fellowship. The authors acknowledge receipt of research funding from the National Institutes of Health, U.S.A. (Grant NS113839); the contents of this article do not represent the views of the United States Government.
Conflict of Interest:
DLH has been a consultant or speaker for Lilly, Amgen, Teva, Intarcia, Merck Sharp & Dohme, and has received research funding from Living Cell Technologies in the past three years.
Abbreviations:
- AM
adrenomedullin
- CGRP
calcitonin gene-related peptide
- CLR
calcitonin receptor-like receptor
- CTR
calcitonin receptor
- GPCR
G protein-coupled receptor
- GWAS
genome-wide association study
- RAMP
receptor activity-modifying protein
References
- 1.Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z, Lifting The Burden: the Global Campaign against H. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bucklan J, Ahmed Z. CGRP antagonists for decreasing migraine frequency: New options, long overdue. Cleve Clin J Med. 2020;87:211–218. [DOI] [PubMed] [Google Scholar]
- 3.Hay DL, Garelja ML, Poyner DR, Walker CS. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br J Pharmacol. 2018;175:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94:1099–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schonauer R, Els-Heindl S, Beck-Sickinger AG. Adrenomedullin - new perspectives of a potent peptide hormone. J Pept Sci. 2017;23:472–485. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan M, Yallampalli U, Dong YL, Hankins GD, Yallampalli C. Expression of adrenomedullin 2 (ADM2)/intermedin (IMD) in human placenta: role in trophoblast invasion and migration. Biol Reprod. 2009;81:777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang SY, Xu MJ, Wang X. Adrenomedullin 2/intermedin: a putative drug candidate for treatment of cardiometabolic diseases. Br J Pharmacol. 2018;175:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hay DL. Amylin. Headache. 2017;57 Suppl 2:89–96. [DOI] [PubMed] [Google Scholar]
- 9.Reda TK, Geliebter A, Pi-Sunyer FX. Amylin, food intake, and obesity. Obes Res. 2002;10:1087–1091. [DOI] [PubMed] [Google Scholar]
- 10.Gadde KM, Allison DB. Long-acting amylin analogue for weight reduction. Lancet. 2021;398:2132–2134. [DOI] [PubMed] [Google Scholar]
- 11.Munoz-Torres M, Alonso G, Raya MP. Calcitonin therapy in osteoporosis. Treat Endocrinol. 2004;3:117–132. [DOI] [PubMed] [Google Scholar]
- 12.Mathiesen DS, Lund A, Vilsboll T, Knop FK, Bagger JI. Amylin and Calcitonin: Potential Therapeutic Strategies to Reduce Body Weight and Liver Fat. Front Endocrinol (Lausanne). 2020;11:617400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poyner DR, Sexton PM, Marshall I, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–246. [DOI] [PubMed] [Google Scholar]
- 14.Bailey RJ, Hay DL. Pharmacology of the human CGRP1 receptor in Cos 7 cells. Peptides. 2006;27:1367–1375. [DOI] [PubMed] [Google Scholar]
- 15.Kuwasako K, Kitamura K, Ito K, et al. The seven amino acids of human RAMP2 (86) and RAMP3 (59) are critical for agonist binding to human adrenomedullin receptors. J Biol Chem. 2001;276:49459–49465. [DOI] [PubMed] [Google Scholar]
- 16.Hay DL, Christopoulos G, Christopoulos A, Poyner DR, Sexton PM. Pharmacological discrimination of calcitonin receptor: receptor activity-modifying protein complexes. Mol Pharmacol. 2005;67:1655–1665. [DOI] [PubMed] [Google Scholar]
- 17.Tilakaratne N, Christopoulos G, Zumpe ET, Foord SM, Sexton PM. Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. J Pharmacol Exp Ther. 2000;294:61–72. [PubMed] [Google Scholar]
- 18.Christopoulos G, Perry KJ, Morfis M, et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol. 1999;56:235–242. [DOI] [PubMed] [Google Scholar]
- 19.Garelja ML, Bower RL, Brimble MA, et al. Pharmacological characterisation of mouse calcitonin and calcitonin receptor-like receptors reveals differences compared with human receptors. Br J Pharmacol. 2022;179:416–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker CS, Eftekhari S, Bower RL, et al. A second trigeminal CGRP receptor: function and expression of the AMY1 receptor. Ann Clin Transl Neurol. 2015;2:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey RJ, Walker CS, Ferner AH, et al. Pharmacological characterization of rat amylin receptors: implications for the identification of amylin receptor subtypes. Br J Pharmacol. 2012;166:151–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohn KJ, Li B, Huang X, et al. CGRP receptor activity in mice with global expression of human receptor activity modifying protein 1. Br J Pharmacol. 2017;174:1826–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamauchi A, Sakurai T, Kamiyoshi A, et al. Functional differentiation of RAMP2 and RAMP3 in their regulation of the vascular system. J Mol Cell Cardiol. 2014;77:73–85. [DOI] [PubMed] [Google Scholar]
- 24.Coester B, Pence SW, Arrigoni S, Boyle CN, Le Foll C, Lutz TA. RAMP1 and RAMP3 Differentially Control Amylin’s Effects on Food Intake, Glucose and Energy Balance in Male and Female Mice. Neuroscience. 2020;447:74–93. [DOI] [PubMed] [Google Scholar]
- 25.Skovbjerg G, Roostalu U, Hansen HH, et al. Whole-brain mapping of amylin-induced neuronal activity in receptor activity-modifying protein 1/3 knockout mice. Eur J Neurosci. 2021. [DOI] [PubMed] [Google Scholar]
- 26.Kraenzlin ME, Ch’ng JL, Mulderry PK, Ghatei MA, Bloom SR. Infusion of a novel peptide, calcitonin gene-related peptide (CGRP) in man. Pharmacokinetics and effects on gastric acid secretion and on gastrointestinal hormones. Regul Pept. 1985;10:189–197. [DOI] [PubMed] [Google Scholar]
- 27.Kim YG, Lone AM, Nolte WM, Saghatelian A. Peptidomics approach to elucidate the proteolytic regulation of bioactive peptides. Proc Natl Acad Sci U S A. 2012;109:8523–8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eftekhari S, Salvatore CA, Johansson S, Chen TB, Zeng Z, Edvinsson L. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res. 2015;1600:93–109. [DOI] [PubMed] [Google Scholar]
- 29.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. [DOI] [PubMed] [Google Scholar]
- 30.Lundblad C, Haanes KA, Grande G, Edvinsson L. Experimental inflammation following dural application of complete Freund’s adjuvant or inflammatory soup does not alter brain and trigeminal microvascular passage. J Headache Pain. 2015;16:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edvinsson L, Nilsson E, Jansen-Olesen I. Inhibitory effect of BIBN4096BS, CGRP(8–37), a CGRP antibody and an RNA-Spiegelmer on CGRP induced vasodilatation in the perfused and non-perfused rat middle cerebral artery. Br J Pharmacol. 2007;150:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kastin AJ, Akerstrom V, Hackler L, Pan W. Adrenomedullin and the blood-brain barrier. Horm Metab Res. 2001;33:19–25. [DOI] [PubMed] [Google Scholar]
- 33.Banks WA, Kastin AJ, Maness LM, Huang W, Jaspan JB. Permeability of the blood-brain barrier to amylin. Life Sci. 1995;57:1993–2001. [DOI] [PubMed] [Google Scholar]
- 34.Edvinsson L, Edvinsson JCA, Haanes KA. Biological and small molecule strategies in migraine therapy with relation to the calcitonin gene-related peptide family of peptides. Br J Pharmacol. 2022;179:371–380. [DOI] [PubMed] [Google Scholar]
- 35.Edvinsson L The Trigeminovascular Pathway: Role of CGRP and CGRP Receptors in Migraine. Headache. 2017;57 Suppl 2:47–55. [DOI] [PubMed] [Google Scholar]
- 36.Edvinsson JCA, Vigano A, Alekseeva A, et al. The fifth cranial nerve in headaches. J Headache Pain. 2020;21:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashina H, Schytz HW, Ashina M. CGRP in human models of primary headaches. Cephalalgia. 2018;38:353–360. [DOI] [PubMed] [Google Scholar]
- 38.Walker CS, Hay DL. CGRP in the trigeminovascular system: a role for CGRP, adrenomedullin and amylin receptors? Br J Pharmacol. 2013;170:1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabrizi M, Bornstein GG, Suria H. Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J. 2010;12:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson KW, Morin SM, Wroblewski VJ, Johnson MP. Peripheral and central nervous system distribution of the CGRP neutralizing antibody [(125)I] galcanezumab in male rats. Cephalalgia. 2019;39:1241–1248. [DOI] [PubMed] [Google Scholar]
- 41.Noseda R, Schain AJ, Melo-Carrillo A, et al. Fluorescently-labeled fremanezumab is distributed to sensory and autonomic ganglia and the dura but not to the brain of rats with uncompromised blood brain barrier. Cephalalgia. 2020;40:229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edvinsson L Role of CGRP in Migraine. Handb Exp Pharmacol. 2019;255:121–130. [DOI] [PubMed] [Google Scholar]
- 43.Rees TA, Hendrikse ER, Hay DL, Walker CS. Beyond CGRP: The calcitonin peptide family as targets for migraine and pain. Br J Pharmacol. 2022;179:381–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wattiez AS, Wang M, Russo AF. CGRP in Animal Models of Migraine. Handb Exp Pharmacol. 2019;255:85–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rea BJ, Davison A, Ketcha MJ, et al. Automated detection of squint as a sensitive assay of sex-dependent calcitonin gene-related peptide and amylin-induced pain in mice. Pain. 2021: 10.1097/j.pain.0000000000002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rea BJ, Wattiez AS, Waite JS, et al. Peripherally administered calcitonin gene-related peptide induces spontaneous pain in mice: implications for migraine. Pain. 2018;159:2306–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avona A, Burgos-Vega C, Burton MD, Akopian AN, Price TJ, Dussor G. Dural Calcitonin Gene-Related Peptide Produces Female-Specific Responses in Rodent Migraine Models. J Neurosci. 2019;39:4323–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wattiez AS, Gaul OJ, Kuburas A, et al. CGRP induces migraine-like symptoms in mice during both the active and inactive phases. J Headache Pain. 2021;22:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun RQ, Lawand NB, Willis WD. The role of calcitonin gene-related peptide (CGRP) in the generation and maintenance of mechanical allodynia and hyperalgesia in rats after intradermal injection of capsaicin. Pain. 2003;104:201–208. [DOI] [PubMed] [Google Scholar]
- 50.Yu LC, Hansson P, Brodda-Jansen G, Theodorsson E, Lundeberg T. Intrathecal CGRP8–37-induced bilateral increase in hindpaw withdrawal latency in rats with unilateral inflammation. Br J Pharmacol. 1996;117:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura-Craig M, Gill BK. Effect of neurokinin A, substance P and calcitonin gene related peptide in peripheral hyperalgesia in the rat paw. Neurosci Lett. 1991;124:49–51. [DOI] [PubMed] [Google Scholar]
- 52.De Logu F, Landini L, Janal MN, et al. Migraine-provoking substances evoke periorbital allodynia in mice. J Headache Pain. 2019;20:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci. 2009;29:8798–8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mason BN, Kaiser EA, Kuburas A, et al. Induction of Migraine-Like Photophobic Behavior in Mice by Both Peripheral and Central CGRP Mechanisms. J Neurosci. 2017;37:204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Avona A, Mason BN, Burgos-Vega C, et al. Meningeal CGRP-Prolactin Interaction Evokes Female-Specific Migraine Behavior. Ann Neurol. 2021;89:1129–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghanizada H, Al-Karagholi MA, Walker CS, et al. Amylin Analog Pramlintide Induces Migraine-like Attacks in Patients. Ann Neurol. 2021;89:1157–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paige C, Plasencia-Fernandez I, Kume M, et al. A female-specific role for Calcitonin Gene-Related Peptide (CGRP) in rodent pain models. J Neurosci. 2022: 10.1523/JNEUROSCI.1137-1521.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tepper SJ. History and Review of anti-Calcitonin Gene-Related Peptide (CGRP) Therapies: From Translational Research to Treatment. Headache. 2018;58 Suppl 3:238–275. [DOI] [PubMed] [Google Scholar]
- 59.Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol. 2010;6:573–582. [DOI] [PubMed] [Google Scholar]
- 60.Edvinsson L, Linde M. New drugs in migraine treatment and prophylaxis: telcagepant and topiramate. Lancet. 2010;376:645–655. [DOI] [PubMed] [Google Scholar]
- 61.Iyengar S, Johnson KW, Ossipov MH, Aurora SK. CGRP and the Trigeminal System in Migraine. Headache. 2019;59:659–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hendrikse ER, Bower RL, Hay DL, Walker CS. Molecular studies of CGRP and the CGRP family of peptides in the central nervous system. Cephalalgia. 2019;39:403–419. [DOI] [PubMed] [Google Scholar]
- 63.Lee Y, Kawai Y, Shiosaka S, et al. Coexistence of calcitonin gene-related peptide and substance P-like peptide in single cells of the trigeminal ganglion of the rat: immunohistochemical analysis. Brain Res. 1985;330:194–196. [DOI] [PubMed] [Google Scholar]
- 64.Eftekhari S, Salvatore CA, Calamari A, Kane SA, Tajti J, Edvinsson L. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience. 2010;169:683–696. [DOI] [PubMed] [Google Scholar]
- 65.Rees TA, Russo AF, O’Carroll SJ, Hay DL, Walker CS. CGRP and the Calcitonin Receptor are Co-Expressed in Mouse, Rat and Human Trigeminal Ganglia Neurons. Frontiers in Physiolgy. 2022:In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. [DOI] [PubMed] [Google Scholar]
- 67.Alpuente A, Gallardo VJ, Asskour L, Caronna E, Torres-Ferrus M, Pozo-Rosich P. Salivary CGRP can monitor the different migraine phases: CGRP (in)dependent attacks. Cephalalgia. 2022;42:186–196. [DOI] [PubMed] [Google Scholar]
- 68.Tvedskov JF, Lipka K, Ashina M, Iversen HK, Schifter S, Olesen J. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol. 2005;58:561–568. [DOI] [PubMed] [Google Scholar]
- 69.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. [DOI] [PubMed] [Google Scholar]
- 70.Olesen J, Diener HC, Husstedt IW, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. [DOI] [PubMed] [Google Scholar]
- 71.Connor KM, Shapiro RE, Diener HC, et al. Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology. 2009;73:970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:382–390. [DOI] [PubMed] [Google Scholar]
- 73.Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014;13:1100–1107. [DOI] [PubMed] [Google Scholar]
- 74.Goadsby PJ, Dodick DW, Ailani J, et al. Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: a double-blind, randomised phase 2b/3 trial. Lancet Neurol. 2020;19:727–737. [DOI] [PubMed] [Google Scholar]
- 75.Croop R, Lipton RB, Kudrow D, et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:51–60. [DOI] [PubMed] [Google Scholar]
- 76.Scheffler A, Schenk H, Wurthmann S, et al. CGRP antibody therapy in patients with drug resistant migraine and chronic daily headache: a real-world experience. J Headache Pain. 2021;22:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alex A, Vaughn C, Rayhill M. Safety and Tolerability of 3 CGRP Monoclonal Antibodies in Practice: A Retrospective Cohort Study. Headache. 2020;60:2454–2462. [DOI] [PubMed] [Google Scholar]
- 78.Hautakangas H, Winsvold BS, Ruotsalainen SE, et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat Genet. 2022;54:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choquet H, Yin J, Jacobson AS, et al. New and sex-specific migraine susceptibility loci identified from a multiethnic genome-wide meta-analysis. Commun Biol. 2021;4:864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scuteri D, Corasaniti MT, Tonin P, Nicotera P, Bagetta G. Role of CGRP pathway polymorphisms in migraine: a systematic review and impact on CGRP mAbs migraine therapy. J Headache Pain. 2021;22:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cargnin S, Pautasso C, Viana M, et al. Association of RAMP1 rs7590387 with the risk of migraine transformation into medication overuse headache. Headache. 2015;55:658–668. [DOI] [PubMed] [Google Scholar]
- 82.Edvinsson L, Grell AS, Warfvinge K. Expression of the CGRP Family of Neuropeptides and their Receptors in the Trigeminal Ganglion. J Mol Neurosci. 2020;70:930–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hendrikse ER, Rees TA, Tasma Z, et al. Calcitonin receptor antibody validation and expression in the rodent brain. Cephalalgia. 2022;Online, ahead of print:3331024221084029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Becskei C, Riediger T, Zund D, Wookey P, Lutz TA. Immunohistochemical mapping of calcitonin receptors in the adult rat brain. Brain Res. 2004;1030:221–233. [DOI] [PubMed] [Google Scholar]
- 85.Liu Y, Beyer A, Aebersold R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell. 2016;165:535–550. [DOI] [PubMed] [Google Scholar]
- 86.Tian Q, Stepaniants SB, Mao M, et al. Integrated genomic and proteomic analyses of gene expression in Mammalian cells. Mol Cell Proteomics. 2004;3:960–969. [DOI] [PubMed] [Google Scholar]
- 87.Garelja ML, Walker CS, Hay DL. CGRP receptor antagonists for migraine. Are they also AMY1 receptor antagonists? Br J Pharmacol. 2022;179:454–459. [DOI] [PubMed] [Google Scholar]
- 88.Walker CS, Raddant AC, Woolley MJ, Russo AF, Hay DL. CGRP receptor antagonist activity of olcegepant depends on the signalling pathway measured. Cephalalgia. 2018;38:437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pan KS, Siow A, Hay DL, Walker CS. Antagonism of CGRP Signaling by Rimegepant at Two Receptors. Front Pharmacol. 2020;11:1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bhakta M, Vuong T, Taura T, Wilson DS, Stratton JR, Mackenzie KD. Migraine therapeutics differentially modulate the CGRP pathway. Cephalalgia. 2021;41:499–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garces F, Mohr C, Zhang L, et al. Molecular Insight into Recognition of the CGRPR Complex by Migraine Prevention Therapy Aimovig (Erenumab). Cell Rep. 2020;30:1714–1723 e1716. [DOI] [PubMed] [Google Scholar]
- 92.ter Haar E, Koth CM, Abdul-Manan N, et al. Crystal structure of the ectodomain complex of the CGRP receptor, a class-B GPCR, reveals the site of drug antagonism. Structure. 2010;18:1083–1093. [DOI] [PubMed] [Google Scholar]
- 93.Amara SG, Arriza JL, Leff SE, Swanson LW, Evans RM, Rosenfeld MG. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science. 1985;229:1094–1097. [DOI] [PubMed] [Google Scholar]
- 94.Schutz B, Mauer D, Salmon AM, Changeux JP, Zimmer A. Analysis of the cellular expression pattern of beta-CGRP in alpha-CGRP-deficient mice. J Comp Neurol. 2004;476:32–43. [DOI] [PubMed] [Google Scholar]
- 95.Manteniotis S, Lehmann R, Flegel C, et al. Comprehensive RNA-Seq expression analysis of sensory ganglia with a focus on ion channels and GPCRs in Trigeminal ganglia. PLoS One. 2013;8:e79523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Henke H, Sigrist S, Lang W, Schneider J, Fischer JA. Comparison of binding sites for the calcitonin gene-related peptides I and II in man. Brain Res. 1987;410:404–408. [DOI] [PubMed] [Google Scholar]
- 97.Kruger L, Mantyh PW, Sternini C, Brecha NC, Mantyh CR. Calcitonin gene-related peptide (CGRP) in the rat central nervous system: patterns of immunoreactivity and receptor binding sites. Brain Res. 1988;463:223–244. [DOI] [PubMed] [Google Scholar]
- 98.Garelja ML, Walker CA, Siow A, et al. Receptor Activity Modifying Proteins Have Limited Effects on the Class B G Protein-Coupled Receptor Calcitonin Receptor-Like Receptor Stalk. Biochemistry. 2018;57:1410–1422. [DOI] [PubMed] [Google Scholar]
- 99.Fraser NJ, Wise A, Brown J, McLatchie LM, Main MJ, Foord SM. The amino terminus of receptor activity modifying proteins is a critical determinant of glycosylation state and ligand binding of calcitonin receptor-like receptor. Mol Pharmacol. 1999;55:1054–1059. [DOI] [PubMed] [Google Scholar]
- 100.Husmann K, Sexton PM, Fischer JA, Born W. Mouse receptor-activity-modifying proteins 1, -2 and -3: amino acid sequence, expression and function. Mol Cell Endocrinol. 2000;162:35–43. [DOI] [PubMed] [Google Scholar]
- 101.Lutz TA. Pancreatic amylin as a centrally acting satiating hormone. Curr Drug Targets. 2005;6:181–189. [DOI] [PubMed] [Google Scholar]
- 102.Gebre-Medhin S, Mulder H, Zhang Y, Sundler F, Betsholtz C. Reduced nociceptive behavior in islet amyloid polypeptide (amylin) knockout mice. Brain Res Mol Brain Res. 1998;63:180–183. [DOI] [PubMed] [Google Scholar]
- 103.Mulder H, Leckstrom A, Uddman R, Ekblad E, Westermark P, Sundler F. Islet amyloid polypeptide (amylin) is expressed in sensory neurons. J Neurosci. 1995;15:7625–7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Russo AF. Overview of Neuropeptides: Awakening the Senses? Headache. 2017;57 Suppl 2:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rees TA, Hay DL, Walker CS. Amylin antibodies frequently display cross-reactivity with CGRP: characterization of eight amylin antibodies. Am J Physiol Regul Integr Comp Physiol. 2021;320:R697–R703. [DOI] [PubMed] [Google Scholar]
- 106.Huang X, Yang J, Chang JK, Dun NJ. Amylin suppresses acetic acid-induced visceral pain and spinal c-fos expression in the mouse. Neuroscience. 2010;165:1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sibilia V, Pagani F, Lattuada N, Rapetti D, Guidobono F, Netti C. Amylin compared with calcitonin: competitive binding studies in rat brain and antinociceptive activity. Brain Res. 2000;854:79–84. [DOI] [PubMed] [Google Scholar]
- 108.Young AA, Vine W, Gedulin BR, et al. Preclinical pharmacology of pramlintide in the rat: Comparisons with human and rat amylin. Drug Develop Res. 1996;37:231–248. [Google Scholar]
- 109.Gingell JJ, Rees TA, Hendrikse ER, et al. Distinct Patterns of Internalization of Different Calcitonin Gene-Related Peptide Receptors. ACS Pharmacol Transl Sci. 2020;3:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Irimia P, Martinez-Valbuena I, Minguez-Olaondo A, et al. Interictal amylin levels in chronic migraine patients: A case-control study. Cephalalgia. 2021;41:604–612. [DOI] [PubMed] [Google Scholar]
- 111.Amylin Pharmaceuticals. SYMLIN®(pramlintide acetate) injection [Package Insert]. In: U.S. Food and Drug Administration., ed. U.S.A.; 2005. [Google Scholar]
- 112.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21:138–167. [DOI] [PubMed] [Google Scholar]
- 113.Gibbons C, Dackor R, Dunworth W, Fritz-Six K, Caron KM. Receptor activity-modifying proteins: RAMPing up adrenomedullin signaling. Mol Endocrinol. 2007;21:783–796. [DOI] [PubMed] [Google Scholar]
- 114.Kis B, Deli MA, Kobayashi H, et al. Adrenomedullin regulates blood-brain barrier functions in vitro. Neuroreport. 2001;12:4139–4142. [DOI] [PubMed] [Google Scholar]
- 115.Ma W, Chabot JG, Quirion R. A role for adrenomedullin as a pain-related peptide in the rat. Proc Natl Acad Sci U S A. 2006;103:16027–16032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fernandez AP, Serrano J, Martinez-Murillo R, Martinez A. Lack of adrenomedullin in the central nervous system results in apparently paradoxical alterations on pain sensitivity. Endocrinology. 2010;151:4908–4915. [DOI] [PubMed] [Google Scholar]
- 117.Huang H, Wang M, Hong Y. Intrathecal administration of adrenomedullin induces mechanical allodynia and neurochemical changes in spinal cord and DRG. Neurosci Lett. 2019;690:196–201. [DOI] [PubMed] [Google Scholar]
- 118.Sugimoto Y, Shiraishi S, Yasuda T, Hamada H, Kawamoto M. Intrathecal adrenomedullin modulates acute inflammatory pain in the rat formalin test. Neurosci Lett. 2013;552:146–150. [DOI] [PubMed] [Google Scholar]
- 119.Petersen KA, Birk S, Kitamura K, Olesen J. Effect of adrenomedullin on the cerebral circulation: relevance to primary headache disorders. Cephalalgia. 2009;29:23–30. [DOI] [PubMed] [Google Scholar]
- 120.Ghanizada H, Iljazi A, Ashina H, et al. Nocebo response in human models of migraine: A systematic review and meta-analysis of randomized, double-blind, placebo-controlled, two-way crossover trials in migraine without aura and healthy volunteers. Cephalalgia. 2021;41:99–111. [DOI] [PubMed] [Google Scholar]
- 121.Ghanizada H, Al-Karagholi MA, Arngrim N, et al. Effect of Adrenomedullin on Migraine-Like Attacks in Patients With Migraine: A Randomized Crossover Study. Neurology. 2021;96:e2488–e2499. [DOI] [PubMed] [Google Scholar]
- 122.Troughton RW, Lewis LK, Yandle TG, Richards AM, Nicholls MG. Hemodynamic, hormone, and urinary effects of adrenomedullin infusion in essential hypertension. Hypertension. 2000;36:588–593. [DOI] [PubMed] [Google Scholar]
- 123.Kita T, Kaji Y, Kitamura K. Safety, Tolerability, and Pharmacokinetics of Adrenomedullin in Healthy Males: A Randomized, Double-Blind, Phase 1 Clinical Trial. Drug Des Devel Ther. 2020;14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hargreaves R, Olesen J. Calcitonin Gene-Related Peptide Modulators - The History and Renaissance of a New Migraine Drug Class. Headache. 2019;59:951–970. [DOI] [PubMed] [Google Scholar]
- 125.Hay DL, Howitt SG, Conner AC, Schindler M, Smith DM, Poyner DR. CL/RAMP2 and CL/RAMP3 produce pharmacologically distinct adrenomedullin receptors: a comparison of effects of adrenomedullin22–52, CGRP8–37 and BIBN4096BS. Br J Pharmacol. 2003;140:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Salvatore CA, Moore EL, Calamari A, et al. Pharmacological properties of MK-3207, a potent and orally active calcitonin gene-related peptide receptor antagonist. J Pharmacol Exp Ther. 2010;333:152–160. [DOI] [PubMed] [Google Scholar]
- 127.Garcia-Martinez LF, Raport CJ, Ojala EW, et al. Pharmacologic Characterization of ALD403, a Potent Neutralizing Humanized Monoclonal Antibody Against the Calcitonin Gene-Related Peptide. J Pharmacol Exp Ther. 2020;374:93–103. [DOI] [PubMed] [Google Scholar]
- 128.Benschop RJ, Gehlert DR, Merchant KM, Shanafelt AB. TREATMENT OF MIGRAINE WITH ANTI-CGRP ANTIBODIES. In: World Intellectual Property Organization, ed. International Bureau; 2007. [Google Scholar]
- 129.Zeller J, Poulsen KR, Abdische YN, Pons J, Collier SJ, Rosenthal. A ANTAGONIST ANTIBODIES DIRECTED AGAINST CALCITONIN GENE-RELATED PEPTIDE AND METHODS USING SAME. In: World Intellectual Property Organization, ed. International Bureau; 2007. [Google Scholar]
- 130.Mulderry PK, Ghatei MA, Spokes RA, et al. Differential expression of alpha-CGRP and beta-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience. 1988;25:195–205. [DOI] [PubMed] [Google Scholar]
- 131.Berman G, Croop R, Kudrow D, et al. Safety of Rimegepant, an Oral CGRP Receptor Antagonist, Plus CGRP Monoclonal Antibodies for Migraine. Headache. 2020;60:1734–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jakate A, Blumenfeld AM, Boinpally R, et al. Pharmacokinetics and safety of ubrogepant when coadministered with calcitonin gene-related peptide-targeted monoclonal antibody migraine preventives in participants with migraine: A randomized phase 1b drug-drug interaction study. Headache. 2021;61:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mullin K, Kudrow D, Croop R, et al. Potential for treatment benefit of small molecule CGRP receptor antagonist plus monoclonal antibody in migraine therapy. Neurology. 2020;94:e2121–e2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Freitag FG, Tolebeyan A, Sivakumar D. CGRP monoclonal antibodies along with CGRP receptor antagonists are safe and effective together and compared to standard of care. In. 63rd Annual Scientific Meeting American Headache Society. Virtual; 2021. [Google Scholar]
- 135.Almeida LS, Castro-Lopes JM, Neto FL, Potes CS. Amylin, a peptide expressed by nociceptors, modulates chronic neuropathic pain. Eur J Pain. 2019;23:784–799. [DOI] [PubMed] [Google Scholar]
- 136.Husmann K, Born W, Fischer JA, Muff R. Three receptor-activity-modifying proteins define calcitonin gene-related peptide or adrenomedullin selectivity of the mouse calcitonin-like receptor in COS-7 cells. Biochem Pharmacol. 2003;66:2107–2115. [DOI] [PubMed] [Google Scholar]


