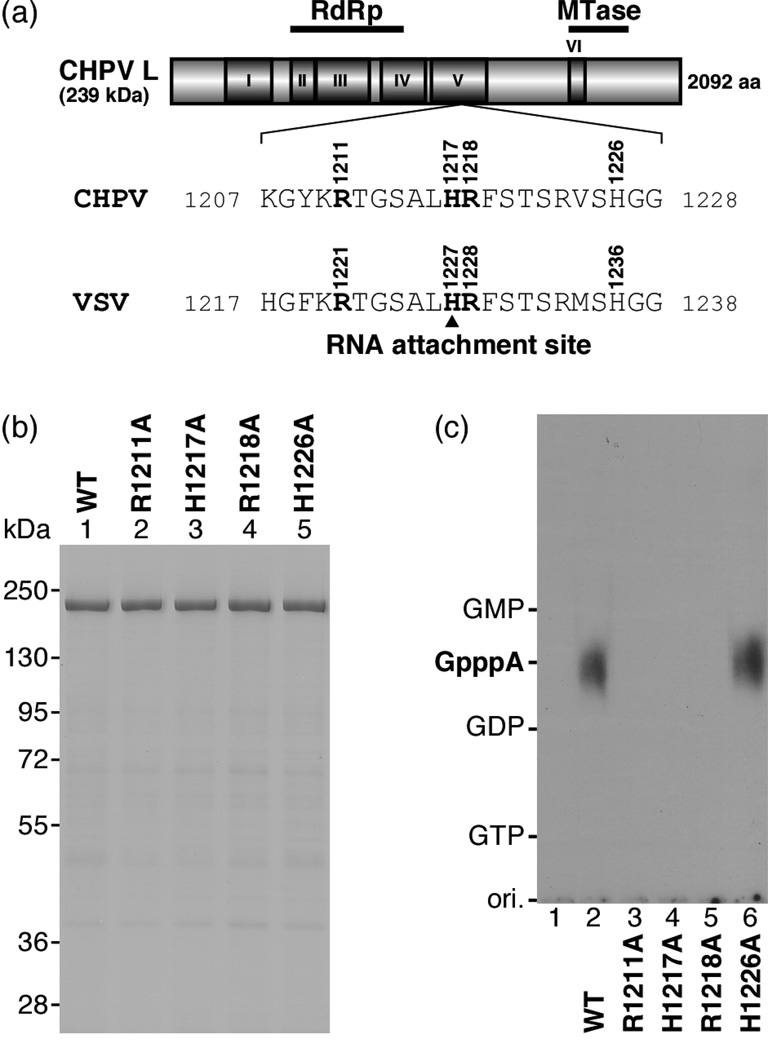
Fig. 2. The HR motif is essential for the PRNTase activity of the CHPV L protein.
(a) A schematic structure of the CHPV L protein (2,092 amino acids) is shown with six amino acid sequence blocks (I–VI) conserved in the NNS RNA viral L proteins. The positions of the putative RNA-dependent RNA polymerase (RdRp) and cap methyltransferase (MTase) domains are indicated on the top. The local sequence containing the HR motif in the CHPV L protein is compared with that in the VSV L protein (H1227, the covalent RNA attachment site).
(b) Wild-type (WT) and mutant CHPV L proteins with indicated amino acid substitutions (0.7 µg) were analyzed by SDS-7.5% PAGE followed by staining with Coomassie Brilliant Blue. The positions of marker proteins are indicated on the left.
(c) The WT and mutant CHPV L proteins (0.3 µg) were subjected to the RNA capping reactions with pppAACAG and [α-32P]GDP as substrates. Lane 1 indicates no L protein.