Figure 4. Effect of treatment withdrawal in resistant colorectal cancers with amplified KRAS G12C.
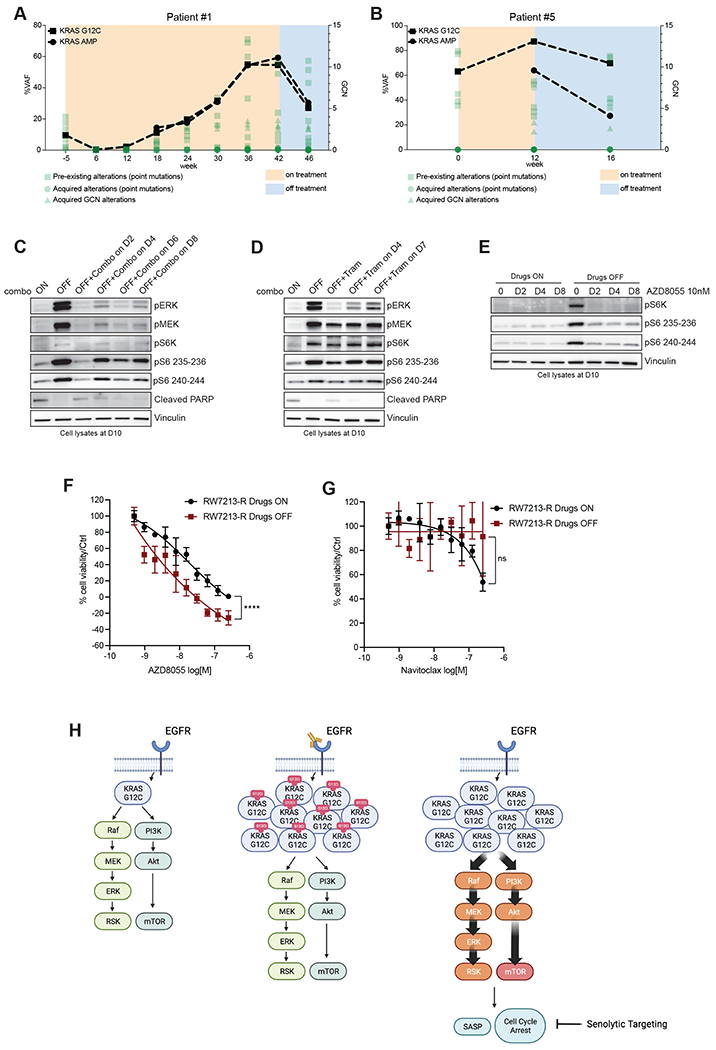
A.Longitudinal analysis of ctDNA in a CRC patient who held KRAS and EGFR inhibition for approximately four weeks after progression. B. Longitudinal analysis of ctDNA in a CRC patient who held KRAS and EGFR inhibition for approximately four weeks after progression. KRAS G12C ctDNA variant allelic frequencies are marked with squares, and KRAS plasma copy numbers are marked with circles. All the other variants are reported in green. C. Western blot analyses of p-ERK, p-MEK, p-S6K, p-S6 and cleaved PARP expression upon drug withdrawal and re-challenge with cetuximab 50μg/mL-sotorasib 3μM combination; vinculin is included as loading control. D. Western blot analyses of p-ERK, p-MEK, p-S6K, p-S6 and cleaved PARP expression upon drug withdrawal and re-challenge with 10nM trametinib; vinculin is included as loading control. E. Western blot analyses of p-S6K and p-S6 upon drug withdrawal or in drug-containing medium after treatment with 10nM AZD8055; vinculin is included as loading control. F. Short term proliferation assay RW7213-R cells in medium containing cetuximab-sotorasib (black) and in senescent conditions (dark red). Cells were seeded in absence or presence of drugs for 4 days and then treated for 96 hours with increasing concentration of AZD8055 and then ATP content was measured using CellTiterGlo. Data represents the average and standard deviation of 3 biological replicates. G. Short term proliferation assay RW7213-R cells in medium containing cetuximab-sotorasib (black) and in senescent conditions (dark red). Cells were seeded in absence or presence of drugs for 4 days and then treated for 96 hours with increasing concentration of Navitoclax and then ATP content was measured using CellTiterGlo. Data represent the average and standard deviation of 3 biological replicates. H. Proposed model: KRAS G12C mutant signaling is maintained at similar level in parental cells and in resistant cells in presence of concomitant EGFR and KRAS G12C blockade. Upon drugs removal, KRAS G12C amplified signaling drives oncogene-induced senescence characterized by elevated mTOR activity creating a new steady state that may be targeted by senolytic treatments.
