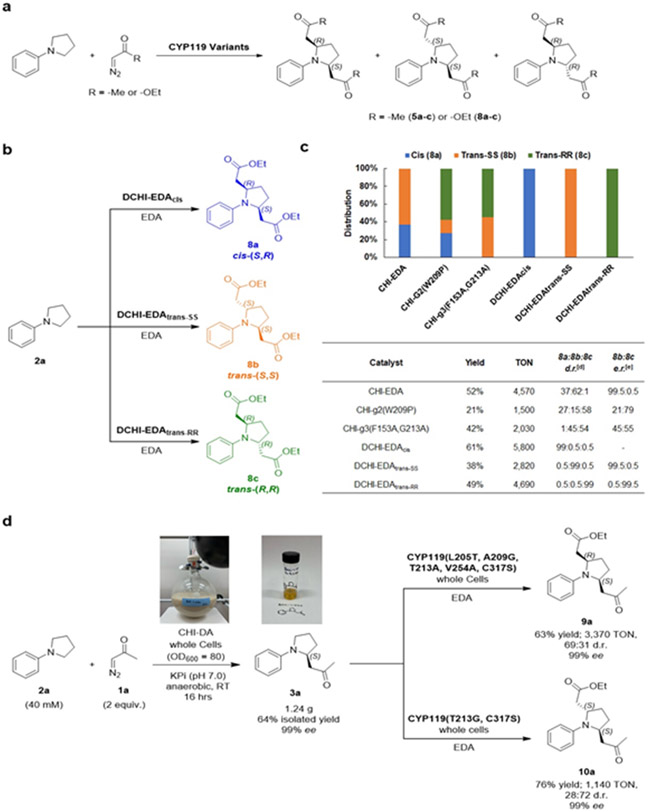
Figure 6. Stereodivergent dual α-C-H functionalization.
(a) General scheme for dual C─H carbene insertion with diazoacetone and ethyl diazoacetate in N-phenylpyrrolidine. (b-c) One-pot dual C─H functionalization of 2a with EDA using CYP119 with stereodivergent selectivity. Whole cell reactions were carried under standard reaction conditions with EDA as in Table 2, but using 40 mM EDA. The graph reports the relative distribution of products 8a-c for the three DCHI-EDA variants along with three other representative CYP119 variants. Assay yields (GC) and TON values reported in the table correspond to the double C─H functionalization product. (d) Tandem dual C─H functionalization of 2a with diazoacetone and EDA. The EDA reaction was carried out using standard reaction conditions as described in Table 2. Diastereomeric and enantiomeric ratios were determined by chiral GC and SFC.