Fig. 7.
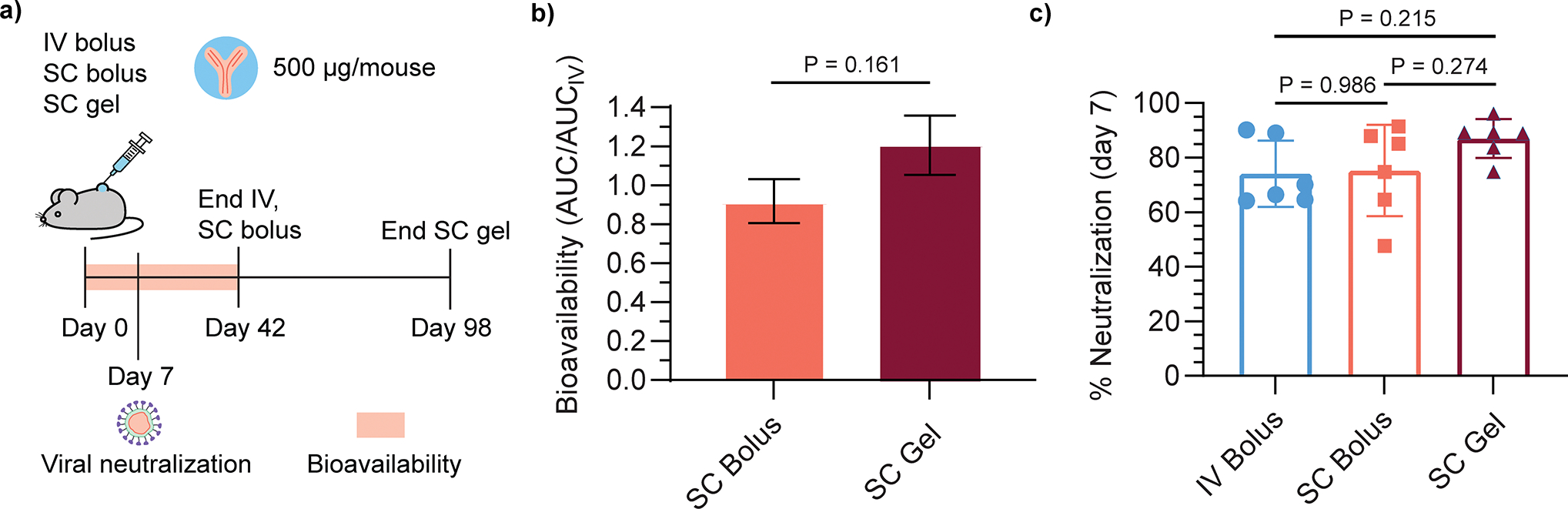
Bioavailability and neutralizing activity of subcutaneously delivered Centi-C10. a) Timecourse of pharmacokinetic study. b) Bioavailability quantified by the area under the curve (AUC) of the Centi-C10 pharmacokinetic profiles by ELISA through day 42 normalized by AUC of the IV bolus group (mean ± SE, n=6 mice/group); p-value determined by two-sided unpaired t-test. c) Neutralizing activity of Centi-C10 from mouse serum 7 days post-administration as determined by spike-pseudotyped lentivirus neutralization assay (mean ± SD, n=6 mice/group, assayed in duplicate). Statistical significance was determined by one-way ANOVA (F (DFn, DFd) = F (2, 15) = 1.906). Tukey post-hoc tests were applied to account for multiple comparisons.
