Abstract
Purpose of review
This review highlights the problem of neuropsychiatric adverse events (AEs) associated with elexacaftor/tezacaftor/ivacaftor (ETI), current suboptimal mitigation approaches, a novel testable mechanistic hypothesis, and potential solutions requiring further research.
Recent findings
Studies show that a minority of PwCF initiating CFTR modulators experience neuropsychiatric AEs including worsening mood, cognition, anxiety, sleep, and suicidality. The GABA-A receptor is a ligand-gated chloride channel, and magnetic resonance spectroscopy neuroimaging studies have shown that reduced GABA expression in rostral anterior cingulate cortex is associated with anxiety and depression. Recent research details the impact of peripheral inflammation and the gut-brain axis on central neuroinflammation. Plasma ETI concentrations and sweat chloride have been evaluated in small studies of neuropsychiatric AEs but not validated to guide dose titration or correlated with pharmacogenomic variants or safety/efficacy.
Summary
Although ETI is well-tolerated by most PwCF, some experience debilitating neuropsychiatric AEs. In some cases, these AEs may be driven by modulation of CFTR and chloride transport within the brain. Understanding biological mechanisms is a critical next step in identifying which PwCF are likely to experience AEs, and in developing evidence-based strategies to mitigate them, while retaining modulator efficacy.
Keywords: cystic fibrosis, CFTR modulator, depression, anxiety, adverse event
INTRODUCTION
In recent years, there has been a revolution in cystic fibrosis (CF) treatment. The drug approach called HEMT (highly effective CF transmembrane conductance regulator [CFTR] modulator therapy), including elexacaftor/tezacaftor/ivacaftor (ETI), has proven transformative for many persons with CF (PwCF), improving lung function and respiratory symptoms, CF-associated morbidity and mortality, and multidimensional quality of life.1–3 ETI does not work mechanistically for up to 10% of PwCF in the US, who have 2 nonsense or other rare CFTR gene variants that do not produce CFTR protein.4 However, ETI is also not a feasible option for some genotype-appropriate PwCF. Despite improvements in physical health, a minority of PwCF initiating CFTR modulators have experienced clinically significant neuropsychiatric adverse effects (AEs), including worsening mood, cognition, anxiety, and sleep, and emergent suicidal thoughts or behavior.5–11 These symptoms can be so profound that some PwCF make the difficult decision to stop using a medication that would reduce their physical suffering and extend their life. In order to develop management strategies for those experiencing biologically-driven neuropsychiatric AEs related to ETI, uncovering underlying mechanisms is an urgent research priority.
In this perspective piece, we first describe the problem of neuropsychiatric AEs associated with ETI and the current suboptimal mitigation approach. Next, we describe a novel testable hypothesis for these ETI-based neuropsychiatric AEs seen in some PwCF, and briefly describe promising scientific approaches to test this hypothesis. Finally, we offer some potential solutions that could result from future research.
THE SCOPE OF THE PROBLEM
There is substantial evidence that ETI can drive clinically significant neuropsychiatric AEs in a subset of PwCF. Our team at Massachusetts General Hospital (MGH) has proposed a conceptual framework regarding etiology and management strategies.9,11 We conducted a retrospective study of symptom trajectories in adults who initiated ETI and subsequently had at least 1 visit with the CF psychiatrist (N=31).11 Of these, 16 PwCF experienced new or worsening neuropsychiatric symptoms that were unexpected and determined to be probably-related to ETI, according to National Cancer Institute guidelines for AE reporting requirements and conservatively considering standard factors such as temporal relationship, response to ETI discontinuation, dose adjustments and rechallenge, and existence of alternative explanation. This represented an 11% incidence of probable neuropsychiatric AEs in the overall cohort of adults taking ETI (N=148) and 52% of the 31 psychiatrically referred adults.11
The literature on ETI effects on depression and anxiety in PwCF evidences a general pattern reflecting increased quality of life for a majority, while a minority has new onset or worsened depression and/or anxiety. Piehler et al. prospectively evaluated CF-related quality of life along with depression and anxiety in 70 adults with CF before and after initiation of ETI.12 At the level of group statistics, this study showed that ETI improved CF-related quality of life, was associated with a very small but statistically significant improvement in median depression scores, and had no effect on median anxiety scores. The authors did not specify the number of PwCF whose depression and anxiety scores increased, but noted that two increased from the moderate to severe range for depression and three increased from the mild to moderate range for anxiety, with uncertain relationship to ETI.12 A retrospective review of 100 adults with CF also measured CF-related quality of life along with depression and anxiety and found no significant group statistical difference in scores before and after starting ETI.13 However, after starting ETI, 22 persons had initiation, increased dose or change in psychiatric medication due to clinical worsening and 23 had new onset of sleep difficulties; two PwCF discontinued ETI due to depression, anxiety, and insomnia.13 In contrast, four PwCF were able to reduce or discontinue psychiatric medication. Quality of life, depression, and anxiety scores were significantly worse in the group that required any psychiatric medication adjustment versus those who did not.13 These results support the MGH study conclusion that a sub-group may be particularly susceptible to mental health side effects.11
Another study of 78 adults taking ETI used a simple (non-validated) survey about the effects of ETI and the COVID-19 pandemic on mental health. Among those taking ETI, 33 (40%) felt COVID-19 contributed to a worsening of either anxiety, depression, or both, and 7 (9%) felt ETI contributed to worsening in their anxiety, depression, or both.7 These results highlight the fact that multiple psychosocial factors can impact mental health. However, studies including the above argue for a unique contribution of ETI in approximately 10%.6,7,11 In 2023, the European Commission added depression as an adverse event with a special warning to the ETI label in the European Union, recommending monitoring for depressed mood, suicidal thoughts and unusual changes in behavior.14 We propose that the neuropsychiatric AEs that occur in a minority of PwCF deserve to be a research priority.
MECHANISTIC HYPOTHESIS
To identify which PwCF are at elevated risk to experience neuropsychiatric AEs and develop strategies to mitigate them, it is essential to elucidate the complex underlying biological mechanisms that may be at play.
CFTR expression in human brain
ETI acts by increasing production of the CFTR gene protein product CFTR and aiding in its functionality at the epithelial surface, where disruption of its ion channel function is thought to be a central mechanism in the failure of mucociliary clearance seen in CF.15 ETI’s mechanism of action centers on supporting the chloride ion channel function of CFTR by targeting the F508del mutation, and by this measure it is very effective. CFTR is classically studied as a chloride (Cl−) channel, and was once thought to be exclusively expressed by epithelial cells, with disruption of its ion channel function in the lung and the gastrointestinal system of PwCF.15 However, more recent research has also found widespread CFTR expression in human brain.16 We hypothesize that neuropsychiatric AEs in some PwCF, perhaps particularly in those with increased baseline inflammation, are driven by modulation of CFTR and chloride transport within the brain.17–19 This is likely related to the fact that chloride is an important ion for normal inhibitory neurotransmission, which plays a central role in controlling anxiety and depression.
The most important inhibitory neurotransmitter in the brain is GABA (γ-aminobutyric acid), and chloride is so central to its proper function that the GABA-A receptor is commonly categorized as a ligand-gated chloride channel. Upon binding of GABA to its receptor, a synaptic pore opens that allows chloride anions to pass, leading to hyperpolarization or inhibition of the neuron. Just as chloride balance dysfunction is a core mechanism of CF, proper chloride balance is a core mechanism of normal brain function, particularly regulation of anxiety and depression. For example, the anti-anxiety and anti-depressive actions of benzodiazepines are exerted by binding to the GABA-A receptor and modulating GABA-induced chloride current.20
Specific emotion regulation brain circuits and structures are particularly reliant upon GABA – and therefore chloride – function. For example, the rostral anterior cingulate cortex (rACC) is an emotion regulation hub whose function is disrupted in multiple psychiatric conditions, including depression and anxiety (See Figure 1). Neuroimaging studies using magnetic resonance spectroscopy (MRS) have repeatedly shown that reduced GABA concentration in rACC is associated with anxiety and depression symptoms.21–24 Loss of GABAergic function disinhibits the excitatory neurotransmitter glutamate, which is thought to be a central mechanism of anxiety and depression. Relatedly, inflammation-related activation of glial cells (the resident immune cells of the central nervous system) causes release of proinflammatory and neuroexcitatory mediators, including glutamate. Therefore, loss of GABA function would interact with inflammatory processes, driving neuropsychiatric consequences.
Figure 1.
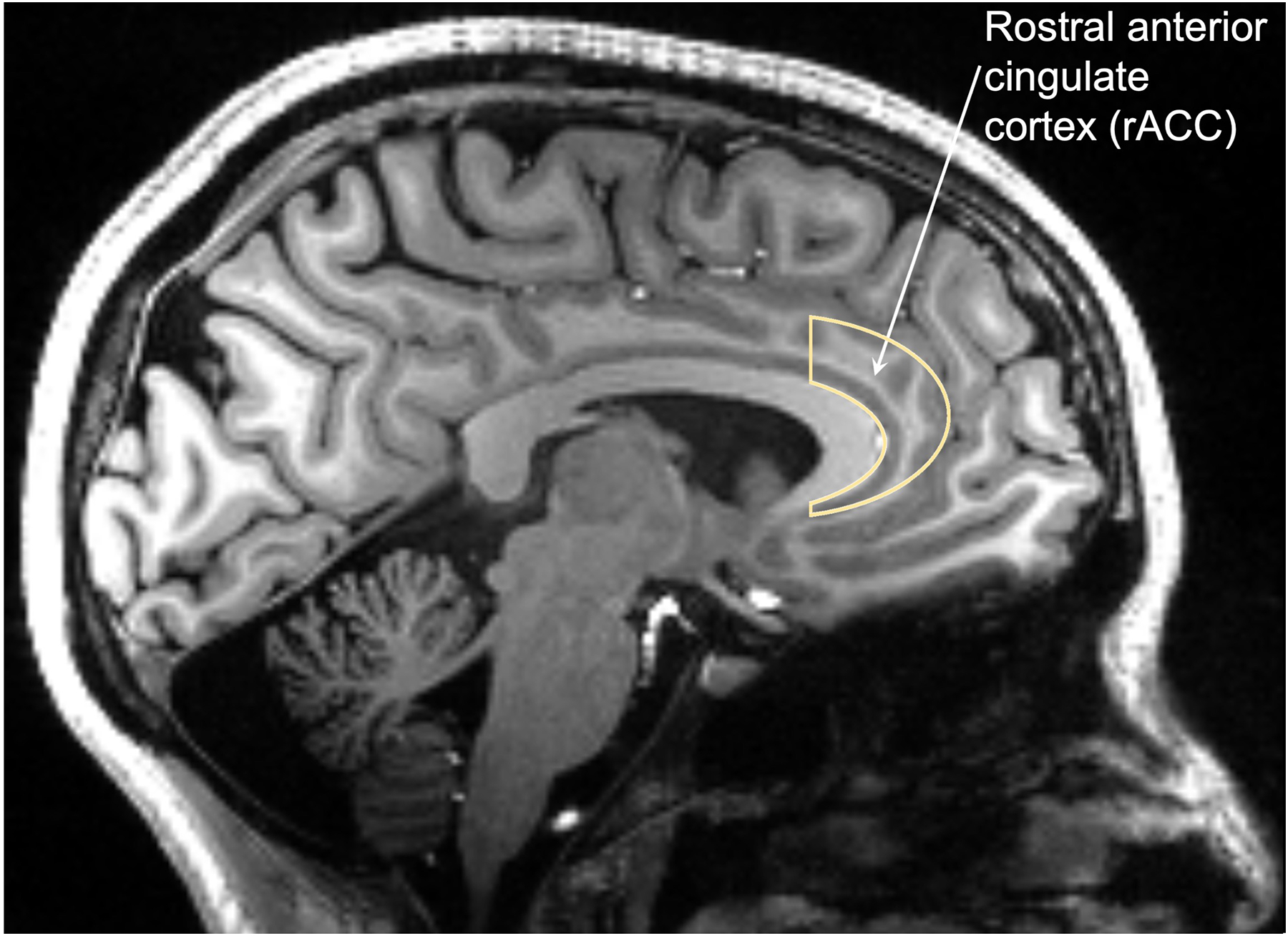
The rostral anterior cingulate cortex (rACC) is an important structure in emotion regulation neurocircuitry. Several studies have shown that decreased GABA concentration in rACC is associated with neuropsychiatric symptoms such as anxiety and depression. ETI drug therapy may affect GABA function via chloride. Furthermore, rACC is dense with serotonin 2A receptors (5HT-2Ar), which are also involved in emotion regulation and directly modulated by ETI.
Furthermore, ETI appears to affect 5-HT2 serotonin receptor subtypes.25 There is high expression of serotonin 2A receptors (5HT-2AR) in the cingulate cortex. This receptor system is intimately related to GABA function both at the neurotransmitter and receptor level.26,27 While this topic demands further study, given the central role of 5-HT2 receptor subtypes in suicidality28 there may be a compounding effect of ETI on both GABA and serotonin (5-HT) function in vulnerable individuals.
Peripheral inflammation can affect the brain
Inflammation may be one of the biological vulnerabilities for the development of neuropsychiatric complications of ETI therapy. Peripheral inflammation is itself a risk factor for depression and anxiety.29 Numerous inflammatory factors are elevated in CF, including IL-1β, TNF-α, IL-630–32 and CRP,33 and even inflammatory cytokine clusters,34 all of which have been associated with depression, psychosis, and generalized anxiety disorder; increases or decreases in these inflammation-related factors can impact neurocognitive functioning across many disease states.35,36 These inflammatory factors have their neuropsychiatric effects by activating glia, which is measurable via specialized neuroimaging techniques.37
Persistent and dysfunctional inflammation in CF extends beyond cytokine production. Blood neutrophils from PwCF display increased phagocytosis, infection-elicited chemotaxis, and intracellular signaling.38 Peripheral blood mononuclear cells, including monocytes, display tolerance to LPS,39 impaired adhesion and trafficking,40 and overly robust generalized inflammatory responses.41 In addition to these hyperinflammatory cellular responses, platelets are highly activated to release proinflammatory lipid mediators,42 all of which can in turn drive inflammation within the subendothelial matrix.43 While inflammatory response improves in PwCF treated with ETI, restoration of CFTR in immune cells and resolution of inflammatory responses can be variable between individuals and it is possible that shifts in inflammatory signals correlate with neuropsychiatric AEs of ETI.44,45
Gut-brain axis in CF
Circulating inflammatory factors are more likely to induce neuroinflammatory consequences if high levels of zonulin, a key regulator of the gut-brain axis,46 are detected in circulation. Zonulin was initially described as a mediator of gastrointestinal permeability by Fasano et al. and is the precursor for haptaglobin-2 (pre-HP).47 Zonulin leads to transactivation of EGF receptor via proteinase-activated receptor 2 (PAR2), resulting in loss of tight junctions’ competency and increased intestinal permeability.47–49 Intact tight junctions are critical for regulation of paracellular trafficking and loss of tight junctions have been associated with numerous inflammatory diseases. Beside regulating gut permeability, zonulin has been shown to also regulate the blood brain barrier (BBB)50 and in a transgenic zonulin mouse model the combined loss of gut and BBB barriers’ function led to behavioral changes that were dependent on intestinal microbiota.51 Gastrointestinal dysbiosis, as seen in celiac disease,50 inflammatory bowel disease,52 acute COVID-19,53 and post-COVID complications,54 have all been associated with increased zonulin release. While zonulin levels have not been reported in CF, dysbiosis in CF is well established.55 CFTR−/− murine models display increased evidence of zonulin-mediated intestinal permeability.56 Importantly, zonulin has been shown to be elevated in numerous mental health conditions,57 including obsessive-compulsive disorder,58 bipolar disorder,59 attention-deficit/hyperactivity disorder,60 and major depressive disorder.61,62 Thus, understanding the role of zonulin in CF and in ETI-mediated neuropsychiatric AEs will be highly informative, and variability in peripheral inflammation and dysbiosis in CF could be important regulators of glial activation in the central nervous system.
Activated glia drive neuroexcitation, the opposite of GABA signaling. If ETI disrupts GABA function, this could predispose some individuals to a double-hit of reduced GABA function (less inhibition) from the drug and intensified glutamate signaling (more excitation) from the inflammation. This effect may potentially be exacerbated in individuals with reduced ability to metabolize ETI, including due to genetic variants.
Pharmacogenomic variation
Decreased drug metabolism may be a factor in driving CNS effects of ETI, particularly given that its components appear capable of crossing the blood-brain barrier. Pharmacogenomic variants in CYP3A4, CYP3A5 (primary metabolism), and additionally, ABCG2, SLOC1B1/1B3, ABCB1 may have a minor role in ETI plasma concentrations.63,64 Ivacaftor also inhibits CYP3A4.65,66 Inflammation has also been shown to inhibit CYP3A.67 PwCF have chronic lung infections associated with chronic inflammation. Inhibition of CYP3A4 by either drug-drug interaction or inflammation results in decreased CYP3A4 metabolism and increased plasma ETI concentrations, which could increase risk of AEs. Therefore, dose reduction is recommended when concomitant use of CYP3A4 inhibitors is necessary. Hepatic injury, cataracts, and hypersensitivity reactions were cited in the product labeling as significant AEs, yet it is unclear which or of these are ETI concentration dependent. The relationship between ETI plasma concentrations and neuropsychiatric AEs has yet to be determined. Additionally, degree of CF liver or kidney disease may have an additive impact on metabolism which was not accounted for in ETI metabolism studies in healthy subjects.66,68,69
CURRENT APPROACHES TO MANAGEMENT
One current approach to mitigating neuropsychiatric AEs of ETI in PwCF is off-label ETI dose reduction. This is suboptimal because there is minimal data informing the approach to dose reduction, and while dose reduction can help mitigate neuropsychiatric symptoms for some, it comes at the cost of an uncertain risk of short- or long-term reduced effectiveness against CF symptoms. PwCF, family caregivers and CF care teams may thus be reluctant to employ this strategy or differ in opinion about its risk/benefit ratio. Additionally, in some cases neuropsychiatric AEs continue unless ETI is discontinued or psychopharmacologic therapies are employed.6,11,70
In a study of 266 adults with CF taking ETI, 19 (7%) reported neuropsychiatric AEs including anxiety, low mood, insomnia, brain fog, and reduced concentration.6 Of these, 13 attempted ETI dose reduction, of whom all also received psychological intervention and six received antidepressants; all maintained clinical efficacy and sweat chlorides in the normal to borderline range. Ten of 13 had improvement or resolution of neuropsychiatric AEs, with post-dose reduction sweat chlorides in the normal or borderline range; two required discontinuation and one switched back to ivacaftor.6 The authors hypothesized that neuropsychiatric AEs were attributable to psychiatric vulnerability, differences in elexacaftor metabolism, and increased systemic CFTR expression.6
In a case series of ten PwCF with new neuropsychiatric symptoms after ETI initiation, including anxiety, irritability, sleep disturbance and/or mental slowness, one discontinued ETI and resumed ivacaftor therapy. Nine underwent dose reduction, using a standardized protocol with serial sweat chloride measurement.70 Mean sweat chlorides were similar on the standard dose (33.4 mmol/L) and the reduced dose (34 mmol/L). While six of the nine had complete resolution of symptoms with dose reduction, three had only partial resolution.70
Sweat chloride concentrations decrease with ETI treatment, and data show a direct relationship between improved pulmonary function and sweat chloride concentrations,71 but to our knowledge neither ETI concentrations nor sweat chloride concentrations have been associated with the occurrence of AEs.72 Additionally, when an AE is associated with ETI, there is not a standard approach to monitoring the balance of safety (avoidance of AEs) and efficacy (pulmonary function, exacerbations, etc.). Plasma concentrations of ETI and sweat chloride concentrations have been evaluated in small studies but are not clinically validated to guide dose titration and are not correlated with safety or efficacy.73–75
MECHANISTIC RESEARCH TO IMPROVE UNDERSTANDING OF NEUROPSYCHIATRIC EFFECTS OF ETI
Research to discover measurable markers that correlate with ETI efficacy and AEs is key to AE mitigation. It is important to investigate the impact of pharmacogenes including ABCG2, SLOC1B1/1B3, ABCB1, CYP3A4, and CYP3A5 variants on ETI concentrations in PwCF to determine if these differences in drug metabolism contribute to AEs. If so, pharmacogenomic testing and measurement of ETI concentrations could be incorporated into routine clinical care to predict risk for AEs and guide protocols for individualized ETI dose adjustment and monitoring. Additional efforts are also needed to define dysbiosis and the inflammatory profiles in individuals who develop complications from ETI, and determine the role of the gut-brain axis.76
Measures of drug function and metabolism can be combined with measures of inflammation and central nervous system GABA function. Magnetic resonance spectroscopy (MRS) is a noninvasive neuroimaging technique that uses MRI (magnetic resonance imaging) scanners; it is capable of detecting the concentration of certain chemical metabolites in brain tissue without the use of injections or radiation. Although GABA is only present at millimolar levels in the human brain, its concentration can be measured with tailored MRS sequences, making MRS an effective technique for noninvasively measuring both GABA and neuroinflammation37,77 and therefore a potentially fruitful technique to test the hypothesis that GABA alterations and inflammation are central to neuropsychiatric side effects in some PwCF taking ETI. MRS studies have repeatedly shown that reduced GABA expression in rACC is associated with anxiety and depression symptoms,21–24 providing evidence that similar mechanisms may be occurring – and measurable – in PwCF experiencing neuropsychiatric AEs. This line of work may ultimately elucidate factors contributing to the elevated prevalence of psychiatric conditions such as depression, anxiety and attention-deficit hyperactivity disorder in PwCF,78 predating the availability of CFTR modulators. Further, improved mechanistic understanding will lay the foundation to determine whether psychopharmacologic treatments employing specific mechanisms of action (including novel agents targeting GABA)79 are preferential for managing various neuropsychiatric AEs related to ETI.11
CONCLUSION
Although ETI is well-tolerated by most PwCF, some experience debilitating neuropsychiatric AEs. Understanding biological mechanisms is a critical next step in identifying which PwCF are likely to experience AEs, and in developing evidence-based, efficient strategies to mitigate them, while retaining modulator efficacy.
KEY POINTS.
A minority of PwCF initiating CFTR modulators such as elexacaftor/tezacaftor/ivacaftor (ETI) experience new or worsening mood/anxiety disorders, cognitive impairment, sleep disturbance, or suicidality.
Chloride balance dysfunction is a core mechanism of both cystic fibrosis and psychiatric disorders including anxiety and depression.
Multiple biological factors may contribute to ETI-related neuropsychiatric adverse events, including inflammation, gut dysbiosis, and individual differences in drug metabolism impacting plasma ETI concentrations.
Understanding these biological mechanisms is key to identifying risk for neuropsychiatric adverse events and management strategies that optimize modulator tolerability and efficacy.
Acknowledgements:
We thank Alessio Fasano for his comments on this manuscript.
Financial support and sponsorship:
Cystic Fibrosis Foundation (GEORGI23Y3; 004760Y122—Tillman; YONKER20A0-KB); National Heart, Lung, and Blood Institute (5K08HL143183; Yonker); PolyBio Research Foundation (VanElzakker)
Conflicts of interest:
MBV reports grants from the National Institutes of Health; and service on the board of directors of PolyBio Research Foundation. EMT reports grants and travel reimbursement from the Cystic Fibrosis Foundation; grants from the National Institutes of Health; and personal fees from Snow Companies. LY reports grants from the Cystic Fibrosis Foundation and grants from the National Heart, Lung, and Blood Institute. EMR reports personal fees from Columbia University for a talk in 2022; personal fees from Imaging Endpoints; grants and travel reimbursement from the National Institutes of Health; grants from the National Science Foundation; and service on the advisory board of Brain Spec. AMG reports personal fees from the Belgian Cystic Fibrosis Foundation/King Baudouin Foundation; grants, personal fees, and travel reimbursement from Cystic Fibrosis Foundation; grants from the Dutch Cystic Fibrosis Foundation; travel reimbursement from the European Cystic Fibrosis Society; travel reimbursement from the French Cystic Fibrosis Society; personal fees from the Italian Cystic Fibrosis Research Foundation; grants from the National Heart, Lung, and Blood Institute; personal fees from Saudi Pediatric Pulmonology Association; grants and personal fees from Vertex Pharmaceuticals; and personal fees from Virginia Commonwealth University.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Griese M, Costa S, Linnemann RW, et al. Safety and Efficacy of Elexacaftor/Tezacaftor/Ivacaftor for 24 Weeks or Longer in People with Cystic Fibrosis and One or More F508del Alleles: Interim Results of an Open-Label Phase 3 Clinical Trial. Am J Respir Crit Care Med 2021;203(3):381–385. DOI: 10.1164/rccm.202008-3176LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren CL, Morgan RL, Oermann C, et al. Cystic Fibrosis Foundation Pulmonary Guidelines. Use of Cystic Fibrosis Transmembrane Conductance Regulator Modulator Therapy in Patients with Cystic Fibrosis. Ann Am Thorac Soc 2018;15(3):271–280. DOI: 10.1513/AnnalsATS.201707-539OT. [DOI] [PubMed] [Google Scholar]
- 3. *.Fajac I, Daines C, Durieu I, et al. Non-respiratory health-related quality of life in people with cystic fibrosis receiving elexacaftor/tezacaftor/ivacaftor. J Cyst Fibros 2023;22(1):119–123. DOI: 10.1016/j.jcf.2022.08.018. [DOI] [PubMed] [Google Scholar]; These findings from 2 pivotal phase 3 trials of ETI demonstrate improvement in a range of CF-specific symptoms and functioning on the Cystic Fibrosis Questionnaire-Revised non-respiratory domains.
- 4.Cystic Fibrosis Foundation. https://www.cff.org/research-clinical-trials/restore-cftr-exploring-treatments-rare-and-nonsense-mutations, accessed on July 25, 2023.
- 5.Heo S, Young DC, Safirstein J, et al. Mental status changes during elexacaftor/tezacaftor / ivacaftor therapy. J Cyst Fibros 2022;21(2):339–343. DOI: 10.1016/j.jcf.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 6. **.Spoletini G, Gillgrass L, Pollard K, et al. Dose adjustments of Elexacaftor/Tezacaftor/Ivacaftor in response to mental health side effects in adults with cystic fibrosis. J Cyst Fibros 2022;21(6):1061–1065. DOI: 10.1016/j.jcf.2022.05.001. [DOI] [PubMed] [Google Scholar]; In a study of 266 adults with CF taking ETI, 19 reported neuropsychiatric AEs and dose reductions were attempted in 13 PwCF. Clinical efficacy was maintained for all, but three PwCF experienced sustained neuropsychiatric AEs despite dose reduction.
- 7. *.Sakon C, Vogt H, Brown CD, et al. A survey assessing the impact of COVID-19 and elexacaftor/tezacaftor/ifavacaftor on both physical and mental health in adults with cystic fibrosis. Pediatr Pulmonol 2022. DOI: 10.1002/ppul.26260. [DOI] [PubMed] [Google Scholar]; A survey of 78 adult PwCF taking ETI assessed the effects of ETI and the COVID-19 pandemic on mental health. Among those taking ETI, 33 (40%) of respondents felt that COVID-19 contributed to a worsening of either anxiety, depression, or both, and 7 (9%) of respondents felt that ETI contributed to a worsening in their anxiety, depression, or both.
- 8.Lyman BC, Seay J, Contreary C, et al. Elexacaftor-tezacaftor-ivacaftor overdose in an adolescent female with cystic fibrosis. Pediatr Pulmonol 2022;57(12):3174–3176. DOI: 10.1002/ppul.26116. [DOI] [PubMed] [Google Scholar]
- 9.Talwalkar JS, Koff JL, Lee HB, et al. Cystic Fibrosis Transmembrane Regulator Modulators: Implications for the Management of Depression and Anxiety in Cystic Fibrosis. Psychosomatics 2017;58(4):343–354. DOI: 10.1016/j.psym.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 10. *.Bathgate CJ, Muther E, Georgiopoulos AM, Smith B, Tillman L, Graziano S, Verkleij M, Lomas P, Quittner A. Positive and negative impacts of elexacaftor/tezacaftor/ivacaftor: Healthcare providers’ observations across US centers. Pediatr Pulmonol. 2023. Jun 2. doi: 10.1002/ppul.26527. Epub ahead of print. [DOI] [PubMed] [Google Scholar]; This is the first national study of the impact of ETI on mental health. It elicited US healthcare provider perceptions positive physical and psychological effects, sleep difficulties, cognitive difficulties, worsening mental health, and concerns about the future and finances.
- 11. *.Baroud E, Chaudhary N, Georgiopoulos AM. Management of neuropsychiatric symptoms in adults treated with elexacaftor/tezacaftor/ivacaftor. Pediatr Pulmonol 2023;58(7):1920–1930. DOI: 10.1002/ppul.26412. [DOI] [PubMed] [Google Scholar]; This retrospective study at a single CF center characterizes the trajectories and pragmatic clinical management of neuropsychiatric symptoms associated with ETI in a psychiatrically referred population.
- 12.Piehler L, Thalemann R, Lehmann C, et al. Effects of elexacaftor/tezacaftor/ivacaftor therapy on mental health of patients with cystic fibrosis. Front Pharmacol 2023;14:1179208. DOI: 10.3389/fphar.2023.1179208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. *.Zhang L, Albon D, Jones M, et al. Impact of elexacaftor/tezacaftor/ivacaftor on depression and anxiety in cystic fibrosis. Ther Adv Respir Dis 2022;16:17534666221144211. DOI: 10.1177/17534666221144211. [DOI] [PMC free article] [PubMed] [Google Scholar]; This retrospective study at a single CF center notes that despite no change in average depression or anxiety scores among adults with CF starting ETI, nearly a quarter experienced worsening requiring escalation of psychopharmacologic therapy and a similar proportion had new onset of sleep disturbance. Findings did not differ by timing of ETI in relation to the COVID-19 pandemic.
- 14.Kaftrio Summary of Product Characteristics. Vertex Pharmaceuticals, Boston, MA. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/kaftrio; 2023. [Google Scholar]
- 15.Hanssens LS, Duchateau J, Casimir GJ. CFTR Protein: Not Just a Chloride Channel? Cells 2021;10(11). DOI: 10.3390/cells10112844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Y, Su M, McNutt MA, Gu J. Expression and distribution of cystic fibrosis transmembrane conductance regulator in neurons of the human brain. J Histochem Cytochem 2009;57(12):1113–20. DOI: 10.1369/jhc.2009.953455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caverly LJ, Riquelme SA, Hisert KB. The Impact of Highly Effective Modulator Therapy on Cystic Fibrosis Microbiology and Inflammation. Clin Chest Med 2022;43(4):647–665. DOI: 10.1016/j.ccm.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopp BT, McCulloch S, Shrestha CL, et al. Metabolomic responses to lumacaftor/ivacaftor in cystic fibrosis. Pediatr Pulmonol 2018;53(5):583–591. DOI: 10.1002/ppul.23972. [DOI] [PubMed] [Google Scholar]
- 19.Murgia F, Gagliano A, Tanca MG, et al. Metabolomic Characterization of Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS). Front Neurosci 2021;15:645267. DOI: 10.3389/fnins.2021.645267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morlock EV, Czajkowski C. Different residues in the GABAA receptor benzodiazepine binding pocket mediate benzodiazepine efficacy and binding. Mol Pharmacol 2011;80(1):14–22. DOI: 10.1124/mol.110.069542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabbay V, Bradley KA, Mao X, et al. Anterior cingulate cortex gamma-aminobutyric acid deficits in youth with depression. Transl Psychiatry 2017;7(8):e1216. DOI: 10.1038/tp.2017.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. *.Ironside M, Moser AD, Holsen LM, et al. Reductions in rostral anterior cingulate GABA are associated with stress circuitry in females with major depression: a multimodal imaging investigation. Neuropsychopharmacology 2021;46(12):2188–2196. DOI: 10.1038/s41386-021-01127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an example of a psychiatric neuroscience study using magnetic resonance spectroscopy (MRS) neuroimaging to demonstrate the strong association between reduced GABA in the rostral anterior cingulate cortex (rACC) and neuropsychiatric symptoms such as depression. Future studies of ETI associated neuropsychiatric side effects in CF may use similar methods to both elucidate the immediate drivers of these side effects as develop measures to predict which PwCF are most likely to experience neuropsychiatric side effects with ETI.
- 23.Kantrowitz JT, Dong Z, Milak MS, et al. Ventromedial prefrontal cortex/anterior cingulate cortex Glx, glutamate, and GABA levels in medication-free major depressive disorder. Transl Psychiatry 2021;11(1):419. DOI: 10.1038/s41398-021-01541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 2011;16(4):383–406. DOI: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gavioli EM, Guardado N, Haniff F, et al. A current review of the safety of cystic fibrosis transmembrane conductance regulator modulators. J Clin Pharm Ther 2021;46(2):286–294. DOI: 10.1111/jcpt.13329. [DOI] [PubMed] [Google Scholar]
- 26.Abi-Saab WM, Bubser M, Roth RH, et al. 5-HT2 receptor regulation of extracellular GABA levels in the prefrontal cortex. Neuropsychopharmacology 1999;20(1):92–6. DOI: 10.1016/S0893-133X(98)00046-3. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Hu L, Liu C, et al. 5-HT2 receptors mediate functional modulation of GABAa receptors and inhibitory synaptic transmissions in human iPS-derived neurons. Sci Rep 2016;6:20033. DOI: 10.1038/srep20033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Underwood MD AV. Disconnect Between Brainstem Serotonin Neurons And Prefrontal Cortex Serotonin Receptors In Suicide. Acta Psychopathologia 2018;0(0). [Google Scholar]
- 29.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008;9(1):46–56. DOI: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nichols DP, Chmiel JF. Inflammation and its genesis in cystic fibrosis. Pediatr Pulmonol 2015;50 Suppl 40:S39–56. DOI: 10.1002/ppul.23242. [DOI] [PubMed] [Google Scholar]
- 31.Meoli A, Eickmeier O, Pisi G, et al. Impact of CFTR Modulators on the Impaired Function of Phagocytes in Cystic Fibrosis Lung Disease. Int J Mol Sci 2022;23(20). DOI: 10.3390/ijms232012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santoft F, Hedman-Lagerlof E, Salomonsson S, et al. Inflammatory cytokines in patients with common mental disorders treated with cognitive behavior therapy. Brain Behav Immun Health 2020;3:100045. DOI: 10.1016/j.bbih.2020.100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matouk E, Nguyen D, Benedetti A, et al. C-Reactive Protein in Stable Cystic Fibrosis: An Additional Indicator of Clinical Disease Activity and Risk of Future Pulmonary Exacerbations. J Pulm Respir Med 2016;6(5):1000375. DOI: 10.4172/2161-105X.1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byrne JF, Healy C, Mongan D, et al. Transdiagnostic inflammatory subgroups among psychiatric disorders and their relevance to role functioning: a nested case-control study of the ALSPAC cohort. Transl Psychiatry 2022;12(1):377. DOI: 10.1038/s41398-022-02142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan N, Chen Y, Xia Y, et al. Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl Psychiatry 2019;9(1):233. DOI: 10.1038/s41398-019-0570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lurie DI. An Integrative Approach to Neuroinflammation in Psychiatric disorders and Neuropathic Pain. J Exp Neurosci 2018;12:1179069518793639. DOI: 10.1177/1179069518793639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VanElzakker MB, Brumfield SA, Lara Mejia PS. Neuroinflammation and Cytokines in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A Critical Review of Research Methods. Front Neurol. 2019. Jan 10;9:1033. doi: 10.3389/fneur.2018.01033. Erratum in: Front Neurol. 2019 Apr 02;10:316. Erratum in: Front Neurol. 2020 Sep 17;11:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yonker LM, Marand A, Muldur S, et al. Neutrophil dysfunction in cystic fibrosis. J Cyst Fibros 2021;20(6):1062–1071. DOI: 10.1016/j.jcf.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.del Fresno C, Gomez-Pina V, Lores V, et al. Monocytes from cystic fibrosis patients are locked in an LPS tolerance state: down-regulation of TREM-1 as putative underlying mechanism. PLoS One 2008;3(7):e2667. DOI: 10.1371/journal.pone.0002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorio C, Montresor A, Bolomini-Vittori M, et al. Mutations of Cystic Fibrosis Transmembrane Conductance Regulator Gene Cause a Monocyte-Selective Adhesion Deficiency. Am J Respir Crit Care Med 2016;193(10):1123–33. DOI: 10.1164/rccm.201510-1922OC. [DOI] [PubMed] [Google Scholar]
- 41.Bruscia EM, Zhang PX, Ferreira E, et al. Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator−/− mice. Am J Respir Cell Mol Biol 2009;40(3):295–304. DOI: 10.1165/rcmb.2008-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ortiz-Munoz G, Yu MA, Lefrancais E, et al. Cystic fibrosis transmembrane conductance regulator dysfunction in platelets drives lung hyperinflammation. J Clin Invest 2020;130(4):2041–2053. DOI: 10.1172/JCI129635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabuchi A, Kuebler WM. Endothelium-platelet interactions in inflammatory lung disease. Vascul Pharmacol 2008;49(4–6):141–50. DOI: 10.1016/j.vph.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Zhang S, Shrestha CL, Robledo-Avila F, et al. Cystic fibrosis macrophage function and clinical outcomes after elexacaftor/tezacaftor/ivacaftor. Eur Respir J 2022. DOI: 10.1183/13993003.02861-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheikh S, Britt RD Jr., Ryan-Wenger NA, et al. Impact of elexacaftor-tezacaftor-ivacaftor on bacterial colonization and inflammatory responses in cystic fibrosis. Pediatr Pulmonol 2023;58(3):825–833. DOI: 10.1002/ppul.26261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veres-Szekely A, Szasz C, Pap D, et al. Zonulin as a Potential Therapeutic Target in Microbiota-Gut-Brain Axis Disorders: Encouraging Results and Emerging Questions. Int J Mol Sci 2023;24(8). DOI: 10.3390/ijms24087548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tripathi A, Lammers KM, Goldblum S, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci U S A 2009;106(39):16799–804. DOI: 10.1073/pnas.0906773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fasano A, Not T, Wang W, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000;355(9214):1518–9. DOI: 10.1016/S0140-6736(00)02169-3. [DOI] [PubMed] [Google Scholar]
- 49.Wang W, Uzzau S, Goldblum SE, et al. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci 2000;113 Pt 24:4435–40. DOI: 10.1242/jcs.113.24.4435. [DOI] [PubMed] [Google Scholar]
- 50.Rahman MT, Ghosh C, Hossain M, et al. IFN-gamma, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochem Biophys Res Commun 2018;507(1–4):274–279. DOI: 10.1016/j.bbrc.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 51. *.Miranda-Ribera A, Serena G, Liu J, et al. The Zonulin-transgenic mouse displays behavioral alterations ameliorated via depletion of the gut microbiota. Tissue Barriers 2022;10(3):2000299. DOI: 10.1080/21688370.2021.2000299. [DOI] [PMC free article] [PubMed] [Google Scholar]; This manuscript tests the hypothesis that gut dysbiosis can regulate brain function using the zonulin-transgenic murine model. The authors show that zonulin-dependent alterations in gut permeability are associated with an altered blood brain barrier integrity, neuroinflammation, and behavioral changes.
- 52.Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res 2020;9. DOI: 10.12688/f1000research.20510.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yazici D, Cagan E, Tan G, et al. Disrupted epithelial permeability as a predictor of severe COVID-19 development. Allergy 2023. DOI: 10.1111/all.15800. [DOI] [PubMed] [Google Scholar]
- 54.Yonker LM, Gilboa T, Ogata AF, et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J Clin Invest 2021;131(14). DOI: 10.1172/JCI149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. **.Caley LR, White H, de Goffau MC, et al. Cystic Fibrosis-Related Gut Dysbiosis: A Systematic Review. Dig Dis Sci 2023;68(5):1797–1814. DOI: 10.1007/s10620-022-07812-1. [DOI] [PubMed] [Google Scholar]; This systematic review identified 38 studies studying gut microbiota relevant to cystic fibrosis and highlights areas relevant to CF-related gut dysbiosis that remain research priorities. The literature confirmed CF-related gut dysbiosis, with associated pro-inflammatory responses, widened tight junctions, and reductions in anti-inflammatory responses.
- 56.Ivanoff AE, Einstein GP, Tulp OL. Experimental Biology 2020 Meeting Abstracts. FASEB J 2020;34(S1):1. DOI: 10.1096/fasebj.2020.34.s1.07600. [DOI] [PubMed] [Google Scholar]
- 57.Asbjornsdottir B, Snorradottir H, Andresdottir E, et al. Zonulin-Dependent Intestinal Permeability in Children Diagnosed with Mental Disorders: A Systematic Review and Meta-Analysis. Nutrients 2020;12(7). DOI: 10.3390/nu12071982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Isik U, Aydogan Avsar P, et al. Serum zonulin and claudin-5 levels in children with obsessive-compulsive disorder. Nord J Psychiatry 2020;74(5):346–351. DOI: 10.1080/08039488.2020.1715474. [DOI] [PubMed] [Google Scholar]
- 59.Zengil S, Laloglu E. Evaluation of Serum Zonulin and Occludin Levels in Bipolar Disorder. Psychiatry Investig 2023;20(4):382–389. DOI: 10.30773/pi.2022.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee SY, Li SC, Yang CY, et al. Gut Leakage Markers and Cognitive Functions in Patients with Attention-Deficit/Hyperactivity Disorder. Children (Basel) 2023;10(3). DOI: 10.3390/children10030513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iordache MM, Tocia C, Aschie M, et al. Intestinal Permeability and Depression in Patients with Inflammatory Bowel Disease. J Clin Med 2022;11(17). DOI: 10.3390/jcm11175121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu H, Wang J, Teng T, et al. Biomarkers of intestinal permeability and blood-brain barrier permeability in adolescents with major depressive disorder. J Affect Disord 2023;323:659–666. DOI: 10.1016/j.jad.2022.11.058. [DOI] [PubMed] [Google Scholar]
- 63.Tsai A, Wu SP, Haseltine E, et al. Physiologically Based Pharmacokinetic Modeling of CFTR Modulation in People with Cystic Fibrosis Transitioning from Mono or Dual Regimens to Triple-Combination Elexacaftor/Tezacaftor/Ivacaftor. Pulm Ther 2020;6(2):275–286. DOI: 10.1007/s41030-020-00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trikafta Package Insert. Vertex Pharmaceuticals, Boston, MA; 2019. [Google Scholar]
- 65.Kalydeco Package Insert. Vertex Pharmaceuticals, Boston, MA; 2017. [Google Scholar]
- 66.van der Meer R, Wilms EB, Sturm R, et al. Pharmacokinetic interactions between ivacaftor and cytochrome P450 3A4 inhibitors in people with cystic fibrosis and healthy controls. J Cyst Fibros 2021;20(5):e72–e76. DOI: 10.1016/j.jcf.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 67.Lenoir C, Daali Y, Rollason V, et al. Impact of Acute Inflammation on Cytochromes P450 Activity Assessed by the Geneva Cocktail. Clin Pharmacol Ther 2021;109(6):1668–1676. DOI: 10.1002/cpt.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rey E, Treluyer JM, Pons G. Drug disposition in cystic fibrosis. Clin Pharmacokinet 1998;35(4):313–29. DOI: 10.2165/00003088-199835040-00004. [DOI] [PubMed] [Google Scholar]
- 69.Kearns GL. Hepatic drug metabolism in cystic fibrosis: recent developments and future directions. Ann Pharmacother 1993;27(1):74–9. DOI: 10.1177/106002809302700117. [DOI] [PubMed] [Google Scholar]
- 70. *.Ibrahim H, Danish H, Morrissey D, et al. Individualized approach to elexacaftor/tezacaftor/ivacaftor dosing in cystic fibrosis, in response to self-reported anxiety and neurocognitive adverse events: A case series. Front Pharmacol 2023;14:1156621. DOI: 10.3389/fphar.2023.1156621. [DOI] [PMC free article] [PubMed] [Google Scholar]; In a case series of ten PwCF with new neuropsychiatric symptoms after ETI initiation, nine underwent dose reduction, using a standardized protocol with serial sweat chloride measurement. While six of the nine had complete resolution of symptoms with dose reduction, three had only partial resolution.
- 71.Nichols DP, Paynter AC, Heltshe SL, et al. Clinical Effectiveness of Elexacaftor/Tezacaftor/Ivacaftor in People with Cystic Fibrosis: A Clinical Trial. Am J Respir Crit Care Med 2022;205(5):529–539. DOI: 10.1164/rccm.202108-1986OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barry PJ, Mall MA, Alvarez A, et al. Triple Therapy for Cystic Fibrosis Phe508del-Gating and -Residual Function Genotypes. N Engl J Med 2021;385(9):815–825. DOI: 10.1056/NEJMoa2100665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pigliasco F, Cafaro A, Stella M, et al. Simultaneous Quantification of Ivacaftor, Tezacaftor, and Elexacaftor in Cystic Fibrosis Patients’ Plasma by a Novel LC-MS/MS Method. Biomedicines 2023;11(2). DOI: 10.3390/biomedicines11020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reyes-Ortega F, Qiu F, Schneider-Futschik EK. Multiple Reaction Monitoring Mass Spectrometry for the Drug Monitoring of Ivacaftor, Tezacaftor, and Elexacaftor Treatment Response in Cystic Fibrosis: A High-Throughput Method. ACS Pharmacol Transl Sci 2020;3(5):987–996. DOI: 10.1021/acsptsci.0c00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ryan KJ, Guimbellot JS, Dowell AE, et al. Quantitation of cystic fibrosis triple combination therapy, elexacaftor/tezacaftor/ivacaftor, in human plasma and cellular lysate. J Chromatogr B Analyt Technol Biomed Life Sci 2022;1213:123518. DOI: 10.1016/j.jchromb.2022.123518. [DOI] [PubMed] [Google Scholar]
- 76. *.Hoppe JE, Wagner BD, Kirk Harris J, Rowe SM, Heltshe SL, DeBoer EM, Sagel SD. Effects of ivacaftor on systemic inflammation and the plasma proteome in people with CF and G551D. J Cyst Fibros. 2022. Nov;21(6):950–958. doi: 10.1016/j.jcf.2022.03.012. Epub 2022 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study analyzed blood samples from 64 individuals with CF (with G551D mutation) at baseline, and 1 and 6 months after initiation of ivacaftor and detected significant changes in changes in inflammatory, lipid digestion, and extracellular matrix proteins, proving insight into extrapulmonary impact of highly effective CFTR modulation.
- 77.Puts NA, Edden RA. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog Nucl Magn Reson Spectrosc 2012;60:29–41. DOI: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. *.Bathgate CJ, Hjelm M, Filigno SS, Smith BA, Georgiopoulos AM. Management of Mental Health in Cystic Fibrosis. Clin Chest Med. 2022. Dec;43(4):791–810. doi: 10.1016/j.ccm.2022.06.014. [DOI] [PubMed] [Google Scholar]; This review describes the presentation and management of mental health conditions commonly occurring in PwCF, including depression, anxiety, trauma, childhood behavioral disorders, sleep, disordered eating, and substance misuse.
- 79. *.Cutler AJ, Mattingly GW, Maletic V. Understanding the mechanism of action and clinical effects of neuroactive steroids and GABAergic compounds in major depressive disorder. Transl Psychiatry 2023;13(1):228. DOI: 10.1038/s41398-023-02514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review highlights preclinical and clinical data that support the association of depression with defects in the GABAergic system of neurotransmission, and standard and novel pharmacotherapeutics targeting this system.


