Abstract
Background and Purpose
The development and validation of methods to stratify the risk of rupture of cerebral aneurysms is highly desired since current treatment risks can exceed the natural risk of rupture. Because unruptured aneurysms are typically treated before they rupture, it is very difficult to connect the proposed risk indices to the rupture of an individual aneurysm. The purpose of this case study was to analyze the hemodynamic environment of a saccular aneurysm of the terminal morphology sub-type that was imaged just prior to its rupture and to test whether the hemodynamic characteristics would designate this particular aneurysm as at high risk.
Methods
A patient-specific computational fluid dynamics model was constructed from 3D rotational angiography images acquired just hours before the aneurysm ruptured. A pulsatile flow calculation was performed and hemodynamic characteristics previously connected to rupture were analyzed.
Results
It was found that the aneurysm had a concentrated inflow stream, small impingement region, complex intra-aneurysmal flow structure, asymmetric flow split from the parent vessel to the aneurysm and daughter branches, and high levels of aneurysmal wall shear stress near the impaction zone.
Conclusions
The hemodynamics characteristics observed in this aneurysm right before its rupture are consistent with previous studies correlating aneurysm rupture and hemodynamic patterns in saccular and terminal aneurysms. This study supports the notion that hemodynamic information may be used to help stratify the rupture risk of cerebral aneurysms.
Introduction
The development and validation of methods to stratify and assess the rupture risk of cerebral aneurysms is highly desired since current treatment risks can exceed the natural risk of rupture (1–3). Earlier studies based on patient-specific computational fluid dynamics (CFD) have compared hemodynamics characteristics between rupture and unruptured saccular aneurysms and found that high rate asymmetric inflows, concentrated inflow jets with small impaction zones, elevated maximum wall shear stress (WSS), and complex unstable flows patterns were correlated with clinical history of previous rupture (4–6). Because unruptured aneurysms are typically treated before they rupture, it is very difficult to connect the proposed risk indices to the rupture of an individual aneurysm. This article presents a case study of a saccular aneurysm of the terminal morphological type that was imaged with 3D angiography a few hours before its rupture. This study provides the rare opportunity of analyzing the hemodynamic environment at the time of rupture and of testing whether previously identified risk indices or characteristics would designate such aneurysm as high risk.
Material and Methods
Clinical and Imaging Data
A 59-year-old female patient presented with headaches and subarachnoid hemorrhage (Fisher III and Hunt-Hess II). CT imaging showed three cerebral aneurysms, two in the right middle cerebral artery and one at the tip of the basilar artery. These images also showed that a 7.5 mm aneurysm on the right middle cerebral artery (MCA) had ruptured. This aneurysm was then treated via endovascular embolization with coils. The basilar artery aneurysm was left untreated and the patient presented a few hours later with a new subarachnoid hemorrhage (SAH Fisher IV). A CT scan showed new blood in the vascular territory corresponding to the basilar artery with intra-ventricular bleeding (Figure 1). No blood in the region of the coiled MCA aneurysm was observed in these images. Thus, it was determined that the second hemorrhage was due to the rupture of the basilar tip aneurysm. Unfortunately, the patient then passed away.
Figure 1.
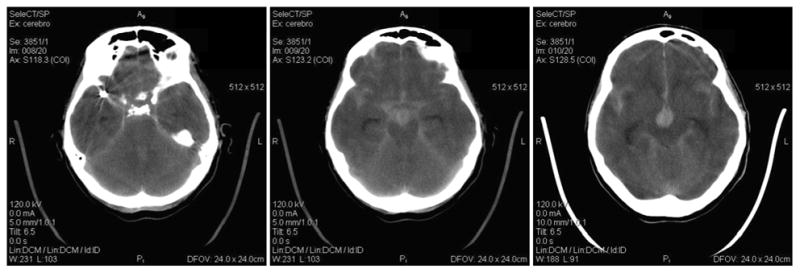
CT images demonstrating the bleeding with intra ventricular hemorrhage of the basilar tip aneurysm and no extra blood in the region of the coiled MCA aneurysm.
During the endovascular procedure, catheter angiograms were performed by standard transfemoral catheterization of the cerebral vessels and digital subtraction imaging was done on an Integris Biplane Unit (Philips Medical Systems, Best, the Netherlands). These images were obtained during a 10-second injection of contrast agent and a 180° rotation with imaging at 15 frames per second for a total of 8 seconds. The corresponding 120 projection images were reconstructed on a dedicated Phillips workstation into a 3D dataset of 256 × 256 × 256 isotropic voxels covering a field of view of 25 mm in all 3 directions. The dataset was exported into a personal computer for vascular and hemodynamic modeling. A 3D rotational angiographic image of the aneurysm is shown in Figure 2 (left panel).
Figure 2.
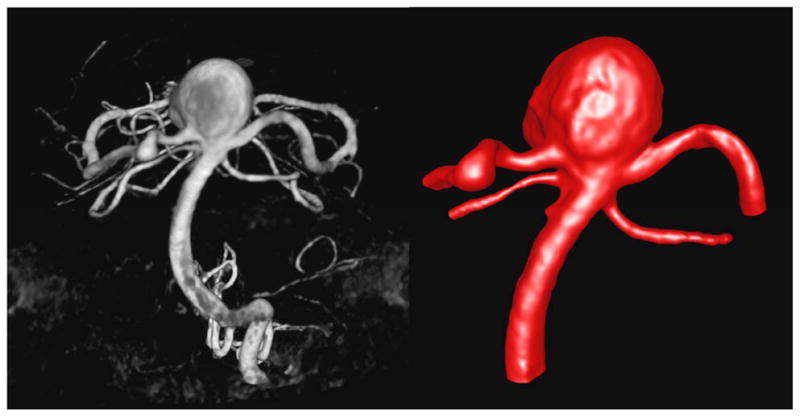
Anterior-posterior view of the 3D rotational angiography of the basilar artery aneurysm before its rupture (left) and corresponding geometrical model (right).
Hemodynamics Modeling
A patient-specific computational fluid dynamics (CFD) model of the aneurysm was constructed from the 3DRA image using previously developed methods (7). The image was filtered and segmentation was performed with a seeded region growing algorithm to reconstruct the topology of the vascular network followed by an iso-surface deformable model to adjust the geometry to the vessel boundaries (8). The vascular model was then smoothed with a non-shrinking algorithm (9), and vessels were truncated perpendicularly to their axis. The final geometrical model obtained is shown in Figure 2 (right panel). A volumetric grid composed of tetrahedral elements was generated using an advancing front method with a resolution of 0.15 mm, resulting in approximately a 3.2 million element mesh. Blood flow was considered an incompressible Newtonian fluid (with density ρ=1.0 g/cm3 and viscosity μ=0.04 Poise) and modeled by the unsteady Navier-Stokes equations in 3D (10). These equations were numerically solved using an implicit finite element formulation on unstructured grids (11, 12). Pulsatile physiologic flow conditions derived from phase-contrast magnetic resonance flow measurements on normal subjects were prescribed at the inlet boundary (basilar artery) using the fully developed Womersley profile and scaled with the inlet area to achieve a mean wall shear stress of 15 dyne/cm2 at the inlet (13, 14). The flow division from the basilar artery to the outflow vessels was determined from their area ratio and corresponding pressure boundary conditions were applied at the model outlets. Vessel walls were assumed rigid and no-slip boundary conditions were applied at the walls. A total of two cardiac cycles were computed using 100 time steps per cycle, the results for the second cycle are presented. Since we are interested in gross characteristics of the blood flow pattern, this number of timesteps is enough.
Data Analysis
The numerical results were visualized and inspected in order to classify the aneurysm according to hemodynamic characteristics previously identified as potential rupture risk factors (6, 15–17). Namely, the following characteristics were considered: a) location of the flow impaction (neck, body or dome); b) size of the flow impaction zone (small – less than 50% of the aneurysm area - or large – more than 50% of the aneurysm area); c) the inflow jet concentration (concentrated or diffuse); d) flow pattern type (simple – single vortical structure – or complex – more than one vortical structures); e) terminal flow pattern type (type A: flow splits from parent vessel into the two daughter branches and the aneurysm, type B: flow splits into one daughter branch and the aneurysm, type C: flow enters the aneurysm before flowing to the two daughter branches). It was verified that the main aneurysm hemodynamics characteristics did not change by varying the inflow conditions for different heart rates. In addition, the maximum WSS at the neck and the aneurysm sac were recorded and the area of the aneurysm under low WSS was calculated. The maximum, minimum and mean WSS of the aneurysm sac were recorded.
Results
Wall Shear Stress and Pressure
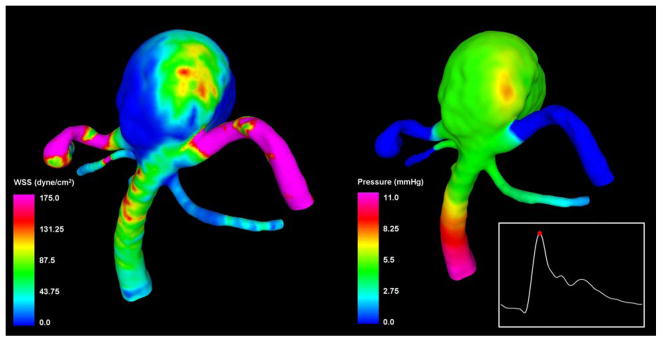
Visualizations of the distributions of hemodynamic forces (wall shear stress and pressure) on the vascular wall at peak systole are presented in Figure 2. These visualizations show that the wall shear stress (Figure 3, left panel) reached a maximum of 393 dyne/cm2 at the neck of the aneurysm and the origin of the left posterior cerebral artery. In the aneurysm sac, the wall shear stress reached a maximum of 160 dyne/cm2 on the left part of the aneurysm body at the zone of impaction of the inflow stream. The size of the flow impingement region (and elevated WSS) was small compared to the aneurysm sac (approximately 20% of the area of the aneurysm sac). The rest of the aneurysm sac was subjected to WSS values lower than those in the parent basilar artery. The pressure was mostly uniform throughout the aneurysm sac, except for a small region around the point of flow impaction, where a small overpressure of approximately 1.5 mmHg can be observed (Figure 3, right panel). Minimum and mean WSS within the aneurysm sac was 0.13 dyne/cm2 and 14.7 dyne/cm2 respectively.
Figure 3.
Visualizations of the distributions of wall shear stress magnitude (left) and pressure (right) at peak systole.
Intra-Aneurysmal Flow Pattern
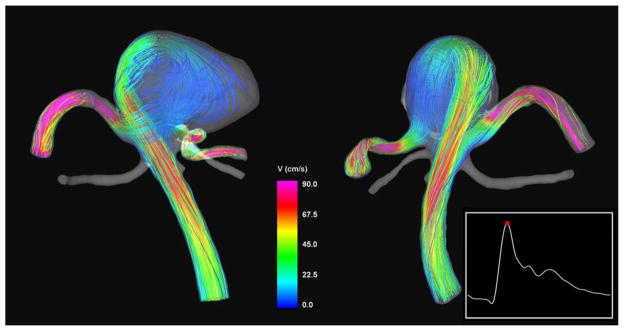
Visualizations of the intra-aneurysmal blood flow structure at peak systole are presented in Figure 4. This figure shows instantaneous streamlines colored with the velocity magnitude, from two viewpoints. It can be seen that the flow stream from the parent vessel splits at the left part of the aneurysm neck, creating two streams, one flowing to the left posterior cerebral artery and the other into the aneurysm. The stream flowing into the aneurysm impacts the aneurysm wall on the left side of the aneurysm body and creates a complex flow pattern with several regions of flow recirculation, before flowing into the right posterior cerebral artery.
Figure 4.
Visualizations of the aneurysm blood flow pattern at peak systole, from two viewpoints. Streamlines are rendered with colors according to the velocity magnitude.
Inflow Stream
The inflow stream was visualized using iso-velocity surfaces and cutting planes. The velocity iso-surface corresponding to v=40 cm/s is presented in Figure 5 (left column) from two viewpoints. The velocity magnitude distributions on two selected cutting planes are also shown in Figure 5 (right column). The locations of the cutting planes are shown together with the iso-velocity surfaces. These visualizations reveal a fairly concentrated inflow jet that impacts on a relatively small region of the aneurysm wall. After impacting the wall, the inflow jet disperses into a wide umbrella-shaped structure along the aneurysm wall, filled with slower vortical structures towards the center of the aneurysm volume.
Figure 5.
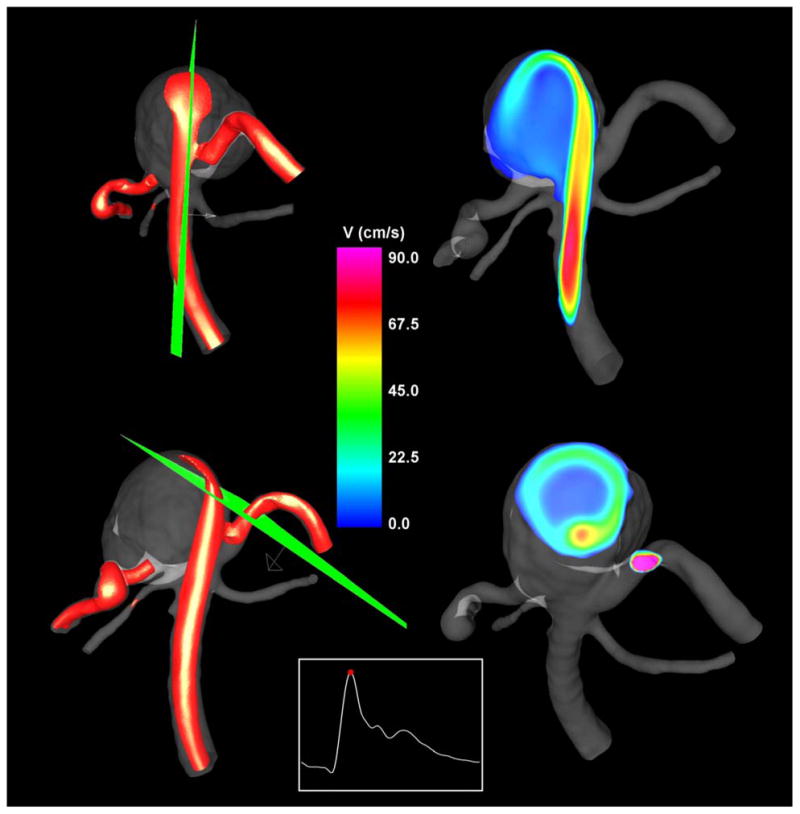
Visualizations of the inflow jet into the aneurysm using iso-velocity surfaces (left column) at peak systole, and velocity magnitudes on plane cuts through the inflow jet (right column). The locations of the cutting planes are displayed together with the velocity iso-surfaces (left column).
Discussion
Previous studies relating hemodynamics and aneurysm rupture have identified some hemodynamic characteristics as potential rupture risk indicators. These studies, along with studies relating geometrical properties of aneurysms and rupture (18–21), were based on the assumption that the rupture event did not substantially affect the aneurysm geometry and therefore that the hemodynamic patterns obtained were representative of the blood flow structures before the rupture. This assumption is common to most studies of aneurysm rupture because unruptured aneurysms are typically treated before they rupture. Thus, it is very difficult to connect the proposed risk indices to the rupture of an individual aneurysm. In the case presented here, we had the rare opportunity of analyzing the hemodynamics in an aneurysm just prior to its rupture and testing whether this analysis would place this particular aneurysm in the high risk categories. Indeed it was observed that this aneurysm had a concentrated inflow jet impacting on a small region of the aneurysm wall, creating a small region of locally elevated wall shear stress near the impaction zone with most of the aneurysm sac under low WSS, and a complex flow pattern inside the aneurysm. These characteristics were previously associated with aneurysm rupture. Additionally, the aneurysm morphology was of the terminal type, and thus it could also be classified according to how the flow stream from the parent vessel divides into the aneurysm and the daughter branches. It was found that the flow stream from the parent vessel split into two streams, one flowing to one of the daughter branches and the other into the aneurysm before flowing to the other daughter branch. This flow division structure has also been previously associated with aneurysm rupture. Finally, the maximum wall shear stress observed in the aneurysm sac (160 dyne/cm2) was closer to the mean wall shear stress in ruptured (188 dyne/cm2) than unruptured (118 dyne/cm2) aneurysms of the terminal morphological type previously studied. All these observations would then designate this aneurysm as at high risk of rupture, and indeed it did go on to bleed. These results are therefore consistent with previous studies relating hemodynamics and aneurysm rupture, and support the notion that hemodynamic characteristics may be used to help stratify the rupture risk of cerebral aneurysms.
Recently, a similar study has been reported (22). In that case, the hemodynamic characteristics in a basilar artery aneurysm just prior to its rupture were described. These characteristics also included a concentrated inflow jet impacting on a small region of the aneurysm wall with an associated complex flow pattern and a region of elevated wall shear stress around the impaction point. However, that aneurysm was fusiform and had a mild stenosis just upstream of the aneurysm which concentrated the flow stream at the aneurysm inlet. The previous studies relating hemodynamics and aneurysm rupture considered mainly saccular aneurysms (15), and focused on the terminal morphological sub-type (6), and on a sub-sample of aneurysms located at the anterior communicating artery (16). In contrast to (22), the current study analyzes the hemodynamic environment just prior to the rupture of a saccular aneurysm with a terminal morphology. Thus, this study adds confirmation that the hemodynamic patterns described are consistent with those most commonly found in aneurysm with a previous history of bleeding.
The current study, however, does not provide information about the actual rupture site or its underlying mechanism. Unfortunately, no pathologic information was available from this case to identify the rupture site and help correlate hemodynamic conditions mode specifically to it. This study only provides information about the hemodynamics environment of the aneurysm just prior to its rupture. Although the characteristics of this hemodynamic environment have been observed to occur more often in aneurysms with a previous history of bleeding, this does not demonstrate that these characteristics are the cause of the rupture. In particular, the local pressure increase at the impingement zone is small compared to the normal intra-arterial pressure variation during the cardiac cycle and may not explain the rupture. Other factors may be involved in the process of rupture such as systemic hypertension or connective tissue deficiencies. Contacts from the peri-aneurysmal environment may cause abnormal damaging stresses and strains related to the vascular pulsatility.
Finally, the current study suffers from several limitations common to most CFD analyses that should be considered when evaluating the results. There are several sources of error that can affect the accuracy of the numerical simulations. The vascular geometry was approximated from 3D angiography image data and may have distortions from the process of construction and image optimization. The flow conditions were not patient-specific, but derived from measurements on normal subjects. The inflow profile was assumed fully developed from representative measurements on normal patients. Several assumptions were made for the CFD calculations including outflow boundary conditions, rigid walls, and Newtonian properties. The wall biomechanics and peri-aneurysmal environment were not considered. However, CFD results have been shown to be robust to small variations of many of these assumptions and approximations (7) and to be able to realistically represent the in vivo hemodynamics (23). It must also be noted that the model construction and meshing took only a few minutes and the pulsatile simulation ran in about 12 hours on a single core of a Silicon Graphics ICE cluster with two quad Xeon 5440 @ 2.83 GHz per node and 16GB of RAM per node. It was also verified that a steady flow calculation, which was completed in two minutes, provided similar qualitative information about the flow characteristics. This suggests that these techniques can potentially be used routinely for patient evaluation during angiography examinations.
Conclusions
This hemodynamic analysis of a saccular intracranial aneurysm of the terminal morphological sub-type that ruptured a few hours after being imaged is consistent with previous studies correlating aneurysm rupture and hemodynamic patterns in saccular and terminal aneurysms. This study adds further support to the notion that hemodynamic information may be used to help stratify the rupture risk of cerebral aneurysms.
Acknowledgments
We thank Philips Medical Systems for financial support. JRC also acknowledges support from The National Institutes of Health grant R01NS059063.
References
- 1.Kassell NF, Torner JC, Haley ECJ, Jane JA, Adams HP, Kongable GL. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg. 1990;73:18–36. doi: 10.3171/jns.1990.73.1.0018. [DOI] [PubMed] [Google Scholar]
- 2.Nishioka H, Torner JC, Graf CJ, Kassell NF, Sahs AL, Goettler LC. Cooperative study of intracranial aneurysms and subarachnoid hemorrhage: a long-term prognostic study. II. Ruptured intracranial aneurysms managed conservatively. Arch Neurol. 1984;41:1142–1146. doi: 10.1001/archneur.1984.04050220036011. [DOI] [PubMed] [Google Scholar]
- 3.White PM, Wardlaw JM. Unruptured intracranial aneurysms. J Neuroradiol. 2003;30:336–350. [PubMed] [Google Scholar]
- 4.Cebral JR, Castro MA, Burgess JE, Pergolizzi RS, Sheridan MJ, Putman CM. Characterization of cerebral aneurysms for assessing risk of rupture by using patient-specific computational hemodynamics models. AJNR Am J Neuroradiol. 2005;26:2550–2559. [PMC free article] [PubMed] [Google Scholar]
- 5.Castro MA, Putman CM, Sheridan MJ, Cebral JR. Hemodynamic patterns of anterior communicating artery aneurysms: a possible association with rupture. AJNR Am J Neuroradiol. 2009;30:297–302. doi: 10.3174/ajnr.A1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro MA, Putman CM, Radaelli A, Frangi AF, Cebral JR. Hemodynamics and rupture of terminal cerebral aneurysms. Acad Radiol. 2009 doi: 10.1016/j.acra.2009.03.022. in press. [DOI] [PubMed] [Google Scholar]
- 7.Cebral JR, Castro MA, Appanaboyina S, Putman CM, Millan D, Frangi AF. Efficient pipeline for image-based patient-specific analysis of cerebral aneurysm hemodynamics: Technique and sensitivity. IEEE TMI. 2005;24:457–467. doi: 10.1109/tmi.2005.844159. [DOI] [PubMed] [Google Scholar]
- 8.Yim PJ, Vasbinder GB, Ho VB, Choyke PL. Isosurfaces as deformable models for magnetic resonance angiography. IEEE Trans Med Imaging. 2003;22:875–881. doi: 10.1109/TMI.2003.815056. [DOI] [PubMed] [Google Scholar]
- 9.Taubin G. A signal processing approach to fair surface design. Proceedings of the 22nd annual conference on Computer graphics and interactive techniques; ACM; 1995. pp. 351–358. [Google Scholar]
- 10.Kundu PK, Cohen IM. Fluid mechanics. Elsevier; 2004. [Google Scholar]
- 11.Cebral JR, Yim PJ, Löhner R, Soto O, Choyke PL. Blood flow modeling in carotid arteries using computational fluid dynamics and magnetic resonance imaging. Acad Radiol. 2002;9:1286–1299. doi: 10.1016/s1076-6332(03)80562-7. [DOI] [PubMed] [Google Scholar]
- 12.Löhner R, Yang C, Cebral JR, et al. Fluid-structure-thermal interaction using adaptive unstructured grids. In: Kvamsdal, editor. Computational Methods for Fluid-Structure Interaction. Tapir Press; 1999. pp. 109–120. [Google Scholar]
- 13.Womersley JR. Method for the calculation of velocity, rate of flow and viscous drag in arteries when the pressure gradient is known. J Physiol. 1955;127:553–563. doi: 10.1113/jphysiol.1955.sp005276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cebral JR, Castro MA, Putman CM, Alperin N. Flow-area relationship in internal carotid and vertebral arteries. Physiol Meas. 2008;29:585–594. doi: 10.1088/0967-3334/29/5/005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cebral JR, Castro MA, Burgess JE, Pergolizzi R, Sheridan MJ, Putman CM. Characterization of cerebral aneurysm for assessing risk of rupture using patient-specific computational hemodynamics models. AJNR Am J Neuroradiol. 2005;26:2550–2559. [PMC free article] [PubMed] [Google Scholar]
- 16.Castro MA, Putman CM, Sheridan MJ, Cebral JR. Hemodynamic patterns of anterior communicating artery aneurysms: a possible association with rupture. AJNR Am J Neuroradiol. 2009;30:297–302. doi: 10.3174/ajnr.A1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valencia A, Guzman AM, Finol EA, Amon CH. Blood flow dynamics in saccular aneurysm models of the basilar artery. J Biomech Eng. 2006;128:516–526. doi: 10.1115/1.2205377. [DOI] [PubMed] [Google Scholar]
- 18.Ujiie H, Tachibana H, Hiramtsu O. Effects of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: a possible index for the surgical treatment of intracranial aneurysms. Neurosurg. 1999;45:119–130. doi: 10.1097/00006123-199907000-00028. [DOI] [PubMed] [Google Scholar]
- 19.Ma B, harbaugh RE, Raghavan ML. Three-dimensional geometrical characterization of cerebral aneurysms. Ann Biomed Eng. 2004;32:264–273. doi: 10.1023/b:abme.0000012746.31343.92. [DOI] [PubMed] [Google Scholar]
- 20.Raghavan ML, Ma B, Harabaugh RE. Quantified aneurysm shape and rupture risk. J Neurosurg. 2005;102:355–362. doi: 10.3171/jns.2005.102.2.0355. [DOI] [PubMed] [Google Scholar]
- 21.Millan D, Dempere-Marco L, Pozo JM, Cebral JR, Frangi AF. Morphological characterization of intracranial aneurysms using 3-D moment invariants. IEEE Trans on Medical Imaging. 2007;26:1270–1282. doi: 10.1109/TMI.2007.901008. [DOI] [PubMed] [Google Scholar]
- 22.Cebral JR, Hendrickson S, Putman CM. Hemodynamics in a lethal basilar artery aneurysm just before its rupture. AJNR Am J Neuroradiol. 2009;30:95–98. doi: 10.3174/ajnr.A1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cebral JR, Pergolizzi R, Putman CM. Computational fluid dynamcis modeling of intracranial aneurysms: qualitatively comparison with cerebral angiography. Acad Radiol. 2007;14:804–813. doi: 10.1016/j.acra.2007.03.008. [DOI] [PubMed] [Google Scholar]