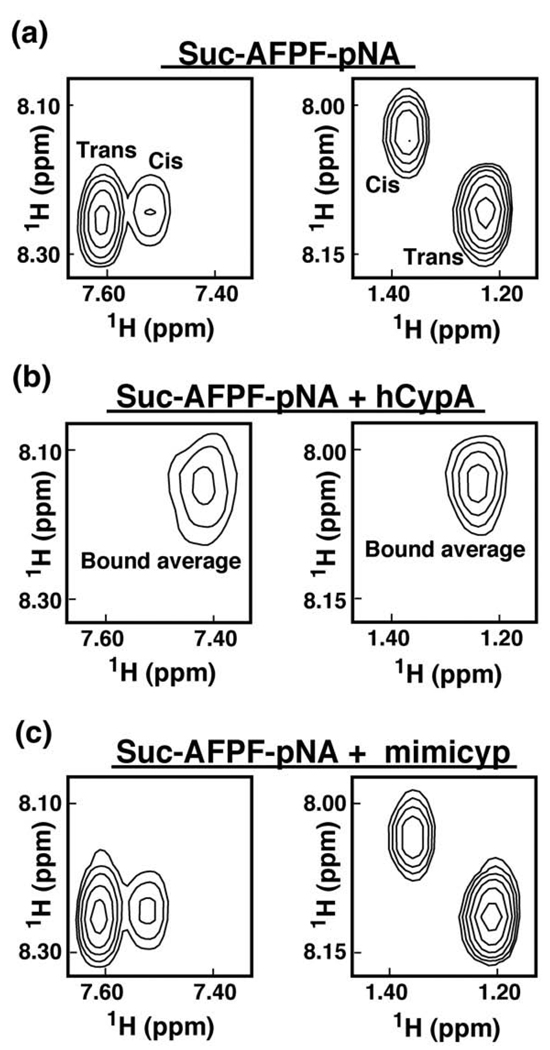
Figure 4.
Mimicyp does not function as a PPIase. 2D-homonuclear TOCSY spectra of 0.5 mM of the proline-containing peptide Suc-AFPF-pNA are shown (a) alone, (b) in the presence of 0.15 mM hCypA, and (c) in the presence of 0.15 mM mimicyp. Resonances corresponding to both pNA H2,5-H3,6 correlations (left panel) and Ala HN-HB correlations (right panel) are shown. In the presence of the active PPIase hCypA there is rapid inter-conversion between these two conformers by the enzyme26 that leads to a single averaged resonance. Mimicyp neither catalyzes nor binds this peptide, as evidenced by the lack of chemical shifts changes.