Fig. 2. Strategies to support rapid optimization cycles.
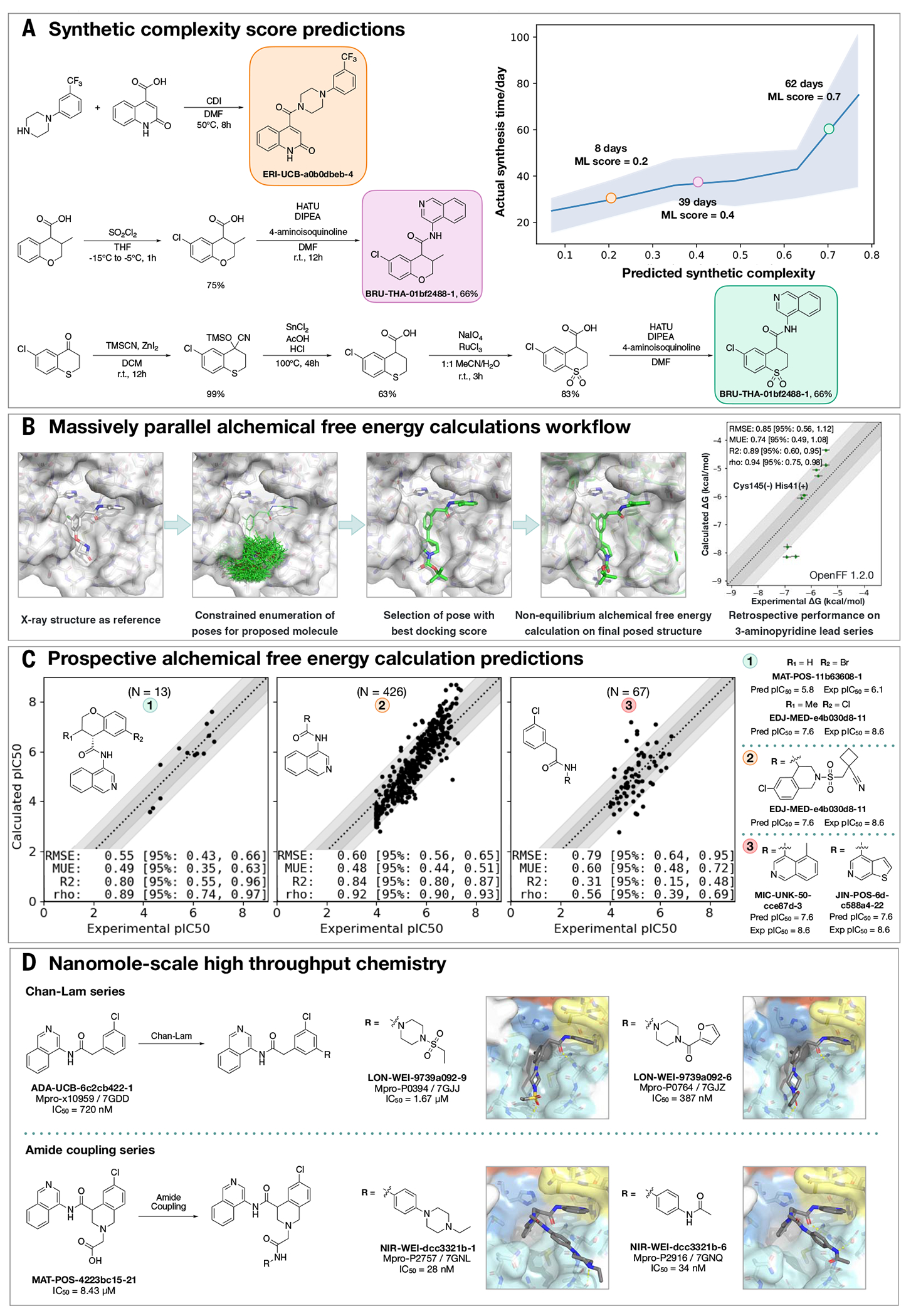
(A) Machine learning forecasts experimental synthesis time (left) and returns efficient routes that leverage more than 10 million in-stock advanced intermediates (right). Our algorithm predicts the probability of each step being successful and predicts synthetic accessibility by taking the product of the probabilities along the whole route. We analyzed all compounds made in COVID Moonshot from 1 May 2020 to 1 July 2021 (n = 898). The right panel exemplifies the experimental execution of the predicted routes, demonstrating the ability of the algorithm to build on functionalized intermediates to shorten synthesis. (B) Applying alchemical free-energy calculations at scale enables us to estimate the potency of compounds. Retrospective assessment of our automated free-energy calculation workflow on early compounds in the 3-aminopyridine series in the first month of the COVID Moonshot campaign suggested that free-energy calculations could provide good predictive utility, which inspired confidence for large-scale deployment during this campaign. Here, the absolute free energy of binding (ΔG) is shown in the rightmost panel by adding a constant offset to the computed relative free-energy differences. (C) Alchemical free-energy predictions for all submissions elaborating on the depicted scaffold for three representative batches of prospective free-energy calculations plotted as calculated (converted using Cheng-Prusoff equation) versus experimental pIC50. Simulations were run using Mpro in dimer form, with neutral catalytic dyad and no restraints. Each batch (numbered 1 to 3 from left to right) is annotated with its scaffold, and top-scoring candidates are shown on the right-hand side (numbered 1 to 3 from top to bottom)–for these, the structure names are shown together with their predicted and experimental pIC50 (“Pred” and “Exp,” respectively). Statistical performance with 95% confidence intervals for each batch is shown as a table in each scatterplot. (D) Two examples of nanomole-scale HTC campaigns used to optimize the potency of intermediate binders, centering on the Chan-Lam reaction (fig. S7) and amide couplings (fig. S8). Direct biochemical screening of crude reactions identified candidates that were resynthesized and in both cases were able to improve the potency of the parent compound. Soaking of crude reaction mixtures of the most potent biochemical hits into Mpro crystals provided complex structures with the identified hits (Chan-Lam PDBs: 7GJJ/7GJZ, resolution: 1.75Å/1.65Å; Amide coupling PDBs: 7GNL/7GNQ, resolution: 1.68Å/1.53Å). In both cases, new interactions were discovered, explaining the improved activity. Although for the Chan-Lam reaction campaign, the extended compounds occupied the intended P4, for the amide-coupling vector, all compounds extended into the P3/5 pockets.
