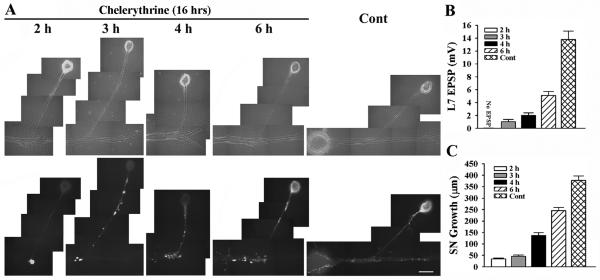
Figure 6.
Blocking PKC activity in sensory neuron and L7 with chelerythrine interferes with initial synapse formation, synapse-associated growth and sensorin expression. A-C. Initial synapse formation, growth and sensorin expression were affected differently when chelerythrine was bath applied to SN-L7 cultures at the indicated times after plating both cells. Co-cultures were imaged after 16 h of drug treatment (18 h to 22 h in culture) after processing for sensorin immunoreactivity (A). Note that the time chelerythrine was added to the co-culture impacted significantly on sensorin expression. The differences in synapse strength and sensorin expression in control cultures at 18 h and 22 h were not significant. An overall ANOVA indicated a significant effect of treatment on sensorin expression in the different compartments (df = 4, 43, F = 82.193, p < 0.001). Individual comparisons indicated that expression in the axon and distal sites were reduced only when inhibitor was added at 2 h (F = 3.825 p < 0.05). Expression in the cell body remained suppressed when inhibitor was added at 3 h and 4 h (F = 32.145, p < 0.01 and F = 30.762, p < 0.01) but reach control levels at 6 h. EPSP amplitude detected after drug treatment was significantly affected (B). Compared to control, adding drug at each time point significantly reduced EPSPs (F = 19.894 to 38.278; p < 0.01). Applying drug after 2 h blocked synapse formation, while adding drug after 6 h allowed cultures to form stronger synapses than those detected when drug was added at 3 h or 4 h (p < 0.05). Extent of growth after drug treatment was also influenced by the time of application (C). For each time point growth was significantly reduced compared to control (F = 13.802 to 92.526; p < 0.01).