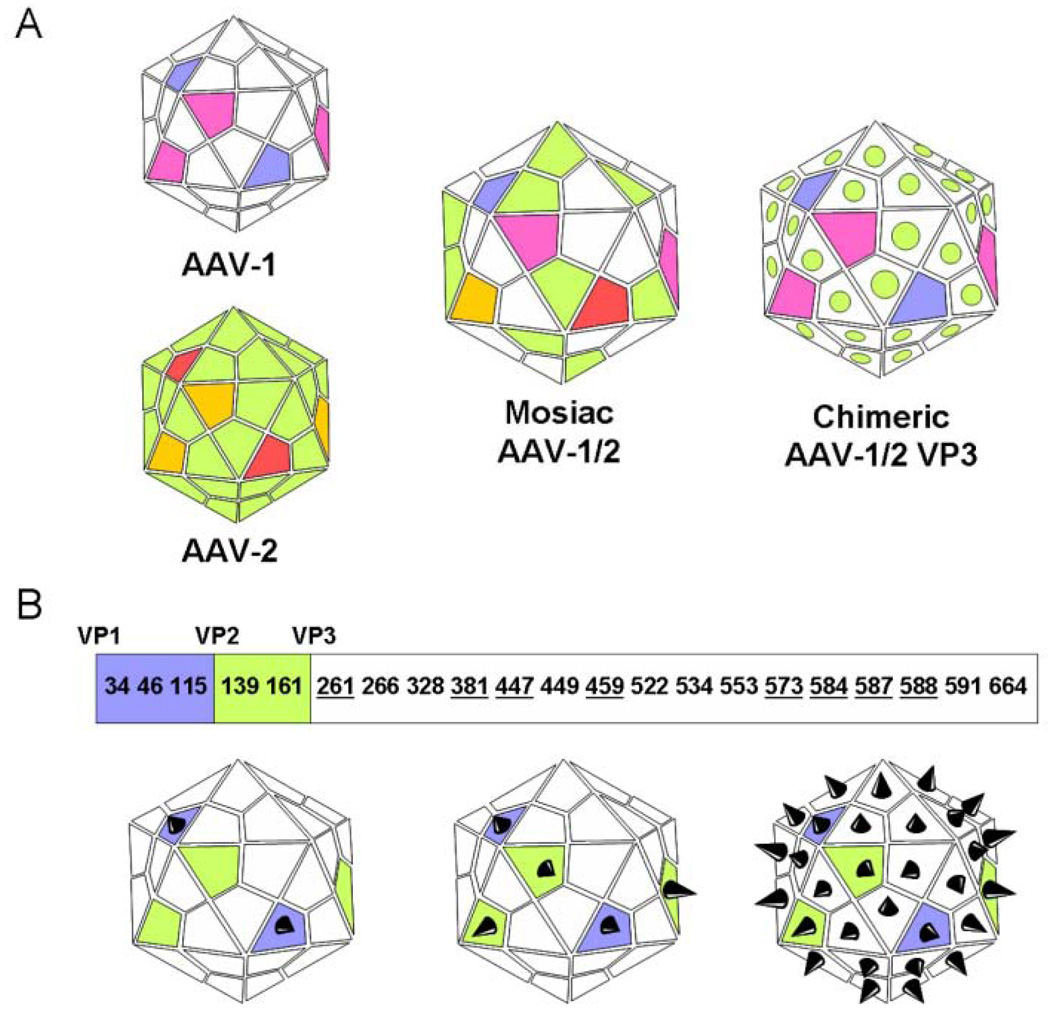
Figure 2.
AAV capsid engineering for enhanced transduction and tissue-specific targeting. (A) Mosaic vectors (capsid structure derived from subunits of different serotypes) or chimeric vectors (capsid proteins modified by domain or amino acid swapping between serotypes) have been generated through trans-capsidation or marker-rescue/domain-swapping (Wu et al., 2006). Seemingly limitless engineered combinations of the 12 identified AAV serotypes and over 100 AAV variants can be generated to enhance tissue targeting and transduction. (B) Insertion of peptide ligands and their presentation on the AAV capsid is a strategy that has been used to enhance targeting and transduction by rAAVs (Muzyczka and Warrington, 2005). The AAV capsid is made up of 3 subunits—VP1 (white), VP2 (green), and VP3 (blue)—in a variable ratio of 1:1:18. Each of these subunits shares a conserved VP3 sequence, with VP2 building upon VP3, and VP1 building upon VP2, as shown. These similarities can be exploited to regulate surface expression of an incorporated peptide. For example, if the peptide sequence is incorporated into the coding section unique to VP1, the peptide will be expressed only by that capsid protein. There is a limited number of sites that can support peptide insertion while maintaining viron infectivity. A 14-residue core RGD peptide motif insertion is possible in VP3 at residues 261, 381, 447, 459, 573, 584, 587, and 588 (Girod et al., 1999; Shi and Bartlett, 2003). Integrin-RGD interactions could be exploited by craniofacial tissue engineers to enhance infection of endothelial cells and localization of rAAV to matrix-laden sites such as bones and teeth. Panel B adapted from Muzyczka and Warrington, 2005.