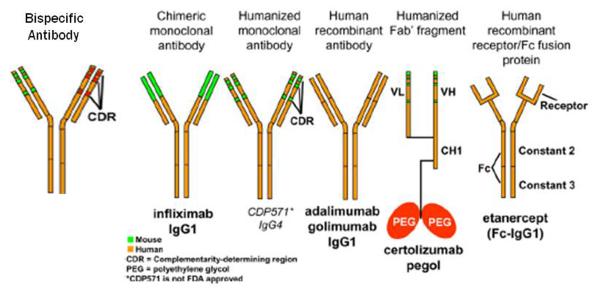
Figure 2.
Standard nomenclature for mAbs identifies their source with the last 4 or 5 letters: -omab, murine: -ximab, chimeric: -zumab, humanized: and –umab, human. The middle part of the name reflects the disease indication for which the mAb was initially intended: -lim for immune and inflammatory diseases, -cir for cardiovascular disorders, and –tu for tumors or neoplastic conditions. The first 3 or 4 letters may be chosen by the sponsor. Modified (by adding the structure of a bispecific antibody) from J Allergy Clin Immunolology, 125(Suppl 2), Lee S.J. et al., Immunomodulator therapy: monoclonal antibodies, fusion proteins, cytokines, and immunoglobulins, p. S314-23, with permission from Elsevier. [28]