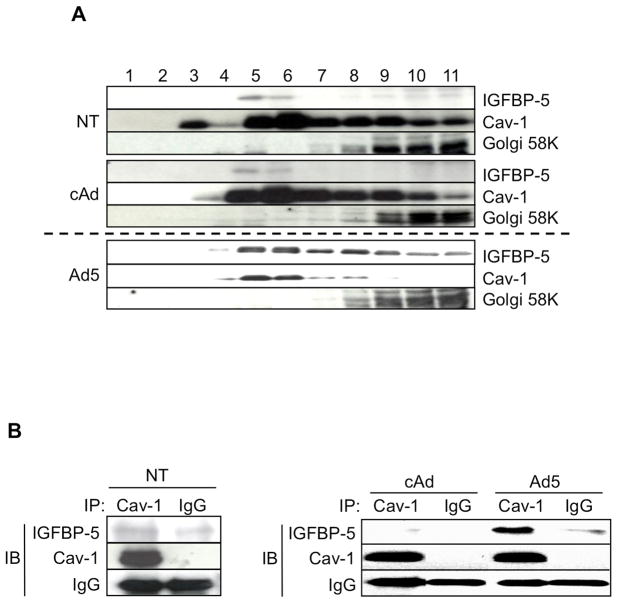
Figure 1.
The localization and binding of Cav-1 and IGFBP-5. A: Caveolae-enriched membrane fractionation was analyzed by immunoblotting for IGFBP-5 and Cav-1. Twelve fractions were obtained from human lung fibroblasts that were infected with cAd, Ad5, or untreated (NT) for 48h using sucrose density gradient fractionation. Eleven fractions (fractions 1–11) were subjected to western blot analysis for IGFBP-5, Cav-1, and 58k Golgi protein as a control for the fractionation. Different exposure times for NT, cAd, and Ad5 are shown as the levels of IGFBP-5 in Ad5 samples were high and the signal was easily saturated. B: Protein-protein interaction between IGFBP-5 and Cav-1 was examined by immunoprecipitation using anti-Cav-1 antibody. Primary lung fibroblasts were non-treated or infected with Ad5 or cAd at an MOI of 50 and cellular lysates were harvested 72 hours post-treatment. Lysates were immunoprecipitated (IP) with anti-Cav-1 antibody or rabbit IgG (IgG). Co-precipitation of IGFBP-5 was examined by immunoblotting (IB). Cav-1 and rabbit immunoglobulins are shown as controls. Left panel; Non-treated cells (NT). Right panel; cAd-or Ad5-infected cells.