Abstract
Increased production of peripheral cytokines and other pro-inflammatory markers has been linked to psychiatric disorders such as major depressive disorder and posttraumatic stress disorder (PTSD). Recent research has pointed to early life stress, particularly childhood maltreatment, as an independent and preventable risk factor for systemic inflammation in adulthood. Some data suggest that adults with a history of childhood maltreatment exhibit a heightened inflammatory response to acute stress challenge. To further elucidate the relationship between childhood maltreatment and pro-inflammatory cytokine production, we examined plasma IL-6 response to the Trier Social Stress Test (TSST) in 69 healthy adult subjects without depression or PTSD. Serial plasma IL-6 concentrations were measured during a standardized psychosocial stressor in n=19 subjects with moderate-severe childhood maltreatment (MAL) and n=50 controls without maltreatment (CTL), as indicated by self-ratings on the Childhood Trauma Questionnaire (CTQ). CTQ total scores were positively correlated with change in overall change in IL-6 response, as well as the maximum IL-6 concentration during the TSST. Greater acute IL-6 release and higher IL-6 concentrations over time were observed for the MAL group relative to the CTL group. Inflammation may be an important developmental mediator linking adverse experiences in early life to poor adult physical and mental health. The results of this preliminary study warrant further investigation in a larger sample.
Keywords: Trier Social Stress Test, stress, cortisol, cytokines, Childhood, Maltreatment, CTQ, IL-6
Introduction
A growing body of research has established that pro-inflammatory cytokines, such as interleukin-6 (IL-6), have systemic effects far beyond the canonical immune response. These immunomodulators have been implicated in a number of psychiatric disorders, particularly major depressive disorder (Groer and Morgan, 2007; Koo and Duman, 2008; O’Brien et al, 2004; Pace et al, 2006) and anxiety disorders (Bauer et al, 2010; Hoge et al, 2009; von Kanel et al, 2007). The pro-inflammatory response is not restricted to pathological states; acute psychological stress produces a transient rise in peripheral cytokines in healthy adult humans (Miller et al, 2005; Steptoe et al, 2007).
However, early life stress, such as childhood maltreatment, appears to be an independent risk for systemic inflammation in otherwise healthy adult humans (Danese et al, 2007). Neuroendocrine-immunological abnormalities established during a stressful childhood are thought to mediate the development of the pro-inflammatory phenotype in adulthood (Chida et al, 2007; Elenkov, 2008; Powell et al, 2009). In a study of stress-induced immune response in men with and without major depressive disorder (MDD), men with MDD and a history of early life stress displayed an exaggerated IL-6 response to an acute psychosocial stressor as compared to non-depressed male subjects (Pace et al, 2006). We previously reported a finding of low cortisol response to stress among adults with exposure to childhood maltreatment (Carpenter et al, 2009), and others have reported an inverse relationship between cytokine release and cortisol release to mild psychological stress challenge (Kunz-Ebrecht et al, 2003). To further elucidate the role that childhood stress exposure plays in the cytokine response to acute stressors encountered in adulthood, we examined the IL-6 response to the Trier Social Stress Test (TSST) in a cohort of healthy adults without psychiatric disorders.
Methods and Materials
Subjects
Subjects were 69 adults (42 women, 27 men) ages 18–64, selected from the community. The subjects in this study were a subset of a larger study cohort that we examined in previous studies (Carpenter et al, 2007; Carpenter et al, 2009). Voluntary written informed consent was obtained from healthy adults who were recruited to participate in a study about stress. The study was approved by the Butler Hospital Institutional Review Board. Subjects who scored in the “moderate” to “severe” range on at least one of the five subscales of the Childhood Trauma Questionnaire (CTQ) comprised the maltreated (MAL) group (n = 19). Subjects scoring “minimal” or “none” on all 5 CTQ subscales, comprised the control group (CTL, n = 50). All subjects were free of pregnancy, significant medical illness, and recreational drug use, as evidenced by complete physical and neurological examination and standard laboratory tests, including electrocardiogram, complete blood count, serum electrolytes, thyroid-stimulating hormone, urine toxicology and urinalysis. Oral contraceptives and estrogen replacement therapies were allowed, and menstrual cycle phase at time of testing was recorded for female subjects. Body mass index (BMI) was calculated as weight (kg) divided by height (meters)2. Exclusion criteria included major medical illness or use of any psychotropic medication or other drug thought to influence HPA axis function (including psychotropic medications, beta blockers, angiotensin-converting enzyme inhibitors, ketoconazole, metyrapone, and corticosteroids). Structured clinical Interview for DSM-IV for Axis I Disorders (SCID-I) was used for psychiatric diagnostic assessments. Diagnoses leading to exclusion included a current or lifetime diagnosis of a primary psychotic disorder or bipolar disorder, current substance dependence or abuse, and current major mood or anxiety disorder. Subjects with prominent personality pathology, as detected through clinical interviews and interactions with research staff during the first two visits, were also excluded. Subjects were remunerated for their time and travel.
Assessment of Maltreatment History, Current Mood/Anxiety Symptoms and Well-Being
In addition to diagnostic interviews, subjects completed a battery of self-report assessments at baseline, including the Inventory for Depressive Symptoms – Self-Rated (IDS-SR; (Rush AJ, 1996), the State-Trait Anxiety Inventory (STAI; (Spielberger, 1983), the Perceived Stress Scale (PSS;(Cohen S, 1983), the Quality of Life - Enjoyment and Satisfaction Questionnaire (QLESQ;(Endicott et al, 1993), and the Childhood Trauma Questionnaire (CTQ; (Bernstein et al, 2003). The CTQ Version 3 is a 28-item self-report instrument comprising five subscales (emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect).
The Acute Stress Protocol
The Trier Social Stress Test (TSST) is a standardized laboratory psychosocial stress protocol that involves public speaking, role-play and mental arithmetic tasks in front of a panel of confederate judges (Kirschbaum et al, 1993). The protocol consists of an anticipation period followed by a 10-minute test period, during which the subject must deliver a monologue speech about his/her qualifications for a chosen vocation and perform mental calculation and recitation of serial subtraction by 13’s. Blood samples, heart rate, and blood pressure data were obtained before, during and after the role-play/arithmetic stressor to monitor for safety and physiologic arousal (−30, 0, +30 and +45 minutes). An intravenous (IV) catheter was established at 11:00 AM to allow time for subjects to accommodate to the biological testing suite environment. Plasma samples were collected via intravenous access at 0 minutes (1:45 PM), and at +15 (2:00 PM), +30 (2:15 PM), +45 (2:30 PM), +60 (2:45 PM), +75 (3:00 PM) and +90 (3:15 PM). Subjects met briefly with the judges immediately after the 0 time point and were told about the public speech role-play, then prepared their speech during the anticipation period (1:50 to 2:00 pm). Subjects were debriefed after the role-pla.y Self-ratings of emotional states, as described elsewhere (Carpenter et al, 2007; Carpenter et al, 2009), were completed at 0, + 30, and +75 minutes. Subjects remained awake in bed for the remainder of the protocol.
Assays
Plasma IL-6 was assayed using high-sensitivity ELISA available from R&D Systems (Minneapolis, MN). Quality assessment samples (concentration, 2.47 pg/ml) have been determined with intra- and inter-assay CVs of 3.3% and 8.9%, respectively.
Statistical Analyses
Analyses were conducted using SPSS 16.0.1. The IL-6 values and age (years) were log10 transformed to fulfill requirements for normal distribution in statistical analyses. All tests were two-tailed with significance defined as p-value <.05. Chi-square and t-test statistics were calculated to compare the two groups on baseline clinical and demographic variables. Baseline variables that differed significantly between groups were entered in post-hoc models as covariates. A final set of post-hoc analyses were performed with estrogen use (n=42) and menstrual cycle phase (n=37) as covariates for women in the sample with available data.
To isolate absolute magnitude of IL-6 rise in response to stress challenge, the summary variable “delta(IL-6)” was calculated as change from time 0 (baseline) to peak IL-6 value after introduction of the stressor. Pair-wise bivariate Pearson correlation matrices were run to evaluate relationships between IL-6 values (time 0, max, and delta) and baseline clinical and demographic variables, including the total CTQ score (continuous data). These were followed by partial correlation analyses, controlling for age and BMI, to examine the relationships between total CTQ score and IL-6.
Two types of general linear models (GLM) were used to examine possible effects of maltreatment group on IL-6 response to the stress test. In the primary analysis, GLM univariate analysis was performed with delta(IL-6) as the dependent variable; group was defined as a fixed factor, and covariates in the model included those found to be statistically related to IL-6 (age and BMI). Post-hoc tests included the same model with the addition of several other covariates, including baseline characteristics that differed between groups and others which theoretically could impact response to acute stress (sub-threshold depression symptoms, state and trait anxiety, level of perceived stress, menstrual cycle phase, and estrogen use). A similarly constructed GLM, with age and BMI as covariates, was also used to examine post-hoc group differences in IL-6 concentration at a single time point (time 0) before exposure to stress in the TSST protocol.
The second analysis strategy used repeated measures GLMs to test effects of maltreatment group on the IL-6 response to the TSST over time. The SPSS GLM repeated measures procedure models dependent variables measured at multiple times using analysis of variance (ANOVA), and the predictor variables may be categorical factors (maltreatment group in our case) or continuous covariates (we included age, BMI, and others). For our models, the dependent variable (IL-6 concentration) was represented by 7 measurement times. Subgroups in the population and covariates are conceived as “control” variables in this analysis. In contrast to the summary variable delta(IL-6), which captures the maximal change in IL-6 concentration during the period of observation (time 0 to 90 minutes), the repeated measures GLM analysis strategy permits comparisons within and between groups during of multiple phases of the response curve over time. The Huynh-Feldt correction was applied when sphericity assumptions were not met.
Results
Bivariate Pearson correlation coefficients revealed significant positive relationships between indices of IL-6 and age, BMI, and CTQ total. No significant correlations emerged for any of the IL-6 measures and sex, depression symptoms, quality of life score, state anxiety, trait anxiety, or perceived stress level. After controlling for age and BMI, we found that CTQ total scores were positively correlated with delta(IL-6) (r=0.33, p=.006) and with max IL-6 (0.37, p=0.002), but not with baseline (time 0) IL-6 (r=0.15, p=0.23).
Clinical and demographic characteristics for the CTL and MAL groups are shown in Table 1. Results of t-tests showed that compared with controls, the group reporting childhood maltreatment was significantly older (t=2.4, p=.03), had a higher mean BMI (t=2.4, p=.03), and higher trait anxiety scores (t=2.1, p=.04). There was a nonsignificant trend (p=.09) toward higher subthreshold depressive symptoms in the maltreated group. State anxiety, perceived stress, and quality of life scores did not differ significantly between groups. The proportion of women did not statistically differ across groups (56% versus 74%; χ2 = .27, p=.14).
Table 1.
Comparison of Clinical and Demographic Characteristics of Groups
| Controls CTL (n = 50) | Maltreated MAL (n = 19) | p | |
|---|---|---|---|
| Age, Mean (SD) years | 24.50 (8.83) | 32.84 (13.89) | 0.03 |
| Range (years) | 18–64 | 18–59 | |
| Gender, n (%) | |||
| Male | 22 (44.0%) | 5 (26.3%) | n.s. |
| Female | 28 (56.0%) | 14 (73.7%) | |
| Body Mass Index, Mean (SD) | 24.61 (3.77) | 28.11 (5.92) | 0.03 |
| Oral Contraceptive or Estrogen Use, N (%of women/group) | 12 (42.8%) | 5 (35.7%) | n.s. |
| Highest Level of Education, N (%) | |||
| Partial High School | 1 (2.0%) | 1 (5.3%) | |
| High School Graduate | 4 (8.0%) | 2 (10.5%) | |
| Technical Degree | 0 (0.0%) | 1 (5.3%) | n.s. |
| Partial College | 27 (54.0%) | 9 (47.4%) | |
| College Graduate | 13 (26.0%) | 4 (21.1%) | |
| Professional Degree | 5 (10.0%) | 2 (10.5%) | |
| Perceived Stress Scale, Mean (SD) | 17.76 (6.70) | 20.37 (6.31) | n.s. |
| IDS-SR Total, Mean (SD) | 8.86 (6.19) | 11.84 (7.24) | 0.09 |
| STAI State Anxiety, Mean (SD) | 30.00 (7.41) | 31.11 (6.54) | n.s. |
| STAI Trait Anxiety, Mean (SD) | 32.41 (8.51) | 37.58 (8.93) | 0.04 |
| CTQ Total score, mean (SD) | 5.98 (0.86) | 10.47 (1.81) | <.01 |
| CTQ Subscales (Categorical), N (%) Moderate-Severe | |||
| Emotional Abuse | 0 (0%) | 11 (57.9%) | |
| Physical Abuse | 0 (0%) | 8 (42.1 %) | |
| Sexual Abuse | 0 (0%) | 4 (21.1%) | |
| Emotional Neglect | 0 (0%) | 9 (47.4%) | |
| Physical Neglect | 0 (0%) | 8 (42.1%) | |
IDS-SR, Inventory of Depressive Symptomatology-Self Report; STAI, State-Trait Anxiety Questionnaire; CTQ, Childhood Trauma Questionnaire. In right column, “n.s.” denotes t-test or chi-square test comparing CTL and MAL groups was not significant at p<.05.
Results of the GLM analysis of delta(IL-6), controlling for age and BMI, showed a significant effect of group (F=8.5, p=.005), such that greater stress-induced increase in IL-6 concentration was associated with a history of maltreatment. The independent effect of maltreatment group remained significant when all other covariates (depression, state and trait anxiety, quality of life, and perceived stress scale scores) were also included the model (F=6.9, p=.01). None of the other variables in the model had a significant effect on delta(IL-6). When menstrual cycle was examined in a subsample of female subjects, maltreatment group retained its significant effect on delta(IL-6), and menstrual phase did not emerge as a significant independent effect. Similarly, the main finding was unchanged when estrogen use was examined as a covariate; estrogen use was not a significant predictor of delta(IL-6). Comparison of IL-6 concentrations immediately before exposure to the stressor (time 0) revealed no significant baseline difference between MAL and CTL groups, after controlling for BMI and age (p=.14).
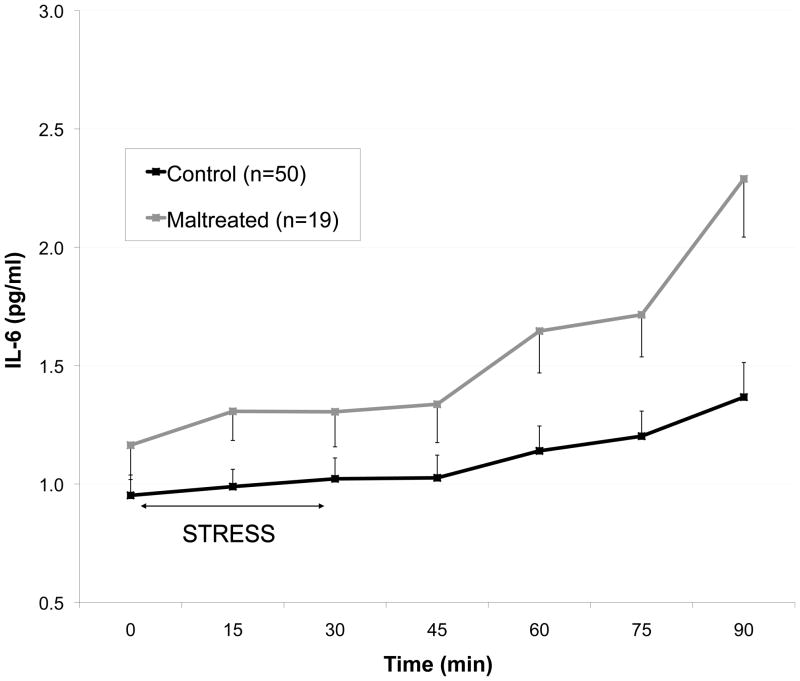
With the repeated measures GLM model, we observed a significant between-groups effect on IL-6 response to TSST (F=4.6, p=.03), after controlling for effects of age and BMI. There was not a significant interaction effect of time X group, indicating that response curve generated by the MAL group was significantly elevated, but essentially parallel to that of the CTL group (Figure 1). When a post-hoc repeated measures model was constructed to include the additional covariates listed above, the independent effect of maltreatment group was reduced to trend level (F=3.3, p=.07), and a trend-level interaction effect of time X group emerged (F=1.9, p=.09). None of the covariates produced a significant effect on lL-6 except BMI (between-subjects effect, F=26.8, p<.01) and depression score (multivariate effect, F=54, p=.02). Estrogen use and menstrual cycle phase did not emerge as significant effects in the repeated measures model.
Fig. 1.
Interleukin-6 (IL-6) response to the Trier Social Stress Test in healthy adults without psychopathology, grouped according to presence or absence of childhood maltreatment. Graph depicts untransformed data, with means adjusted for age and BMI.
Discussion
Previous studies found that subjects with MDD exhibit an exaggerated IL-6 response to acute psychosocial stress (Miller et al, 2005; Weinstein et al, 2010). However, the role of early life environment in the stress-related inflammatory response has not been established. Pace et al (2006) demonstrated that men with MDD reporting childhood maltreatment were found to have a greatly increased IL-6 response to the TSST. The present study sought to clarify the role of early life stress in mediating the IL-6 response, and to disentangle the effects of MDD and early-life maltreatment by assessing the acute inflammatory response in adults without depression. The results of this small pilot study indicate that adverse early environment, measured here by self-reported childhood abuse or neglect, is significantly linked to pro-inflammatory response to acute psychosocial stress in otherwise healthy adults. Subjects in our sample with childhood maltreatment showed greater overall peripheral release of IL-6 during a standard stress challenge, and significantly elevated IL-6 concentrations throughout the TSST observation period, as compared to the control group.
Significant group differences in age and BMI were a limitation of this study. Recent studies have demonstrated that exposure to early life stress increases adult obesity (Boynton-Jarrett et al, 2010; D’Argenio et al, 2009), while others have reported a relationship between BMI and cytokine response to stress (Benson et al, 2009; Brydon et al, 2008). The correlation between BMI and early life stress was also robust in our sample (r=.41, p<.01). Our finding of heightened IL-6 response in the maltreatment group emerged after statistically controlling for effects of BMI (and age). However, insufficient power and collinearity between the variables precluded our ability to elucidate a possible interaction or mediating effect of BMI and childhood maltreatment in the generation of plasma IL-6 responses. Future investigation into this question, with larger groups of subjects matched by age and BMI, will be an important next step for unraveling the relationships among adverse early life environment, obesity, and inflammatory response to acute stress. A larger sample size will also permit examination of different subtypes of childhood maltreatment as independent predictors of cytokine response to stress.
The clinical consequences of having an exaggerated cytokine response to stress are not clear, particularly in healthy adults without major medical disorders. IL-6 is produced by numerous tissues in the body, and identifying the original source of IL-6 measured in plasma is not a straightforward process. In the periphery, IL-6 is produced by leukocytes, skeletal myocytes (Keller et al, 2005), adipocytes (Fried et al, 1998), and splenocytes (Merlot et al, 2004). Animal models have demonstrated that exposure to acute stress increases the expression of pro-inflammatory cytokines both in the periphery (LeMay et al, 1990; Zhou et al, 1993) and within the central nervous system (Butterweck et al, 2003; Suzuki et al, 1997). While there is evidence that peripheral cytokines can be transported across the blood-brain barrier (Threlkeld et al, 2010), the majority of relevant human studies have assessed basal or stimulated IL-6 production in the periphery, likely painting an incomplete picture of the dynamic interaction between the immune system and other physiological systems.
The mechanism by which cytokine response to acute stress might lead to development of mental and physical health disorders is unknown, but the findings of this study and the work of other research groups (Benson et al, 2009; Chen et al, 2006) provide some support for the notion that individuals with this phenotype may be predisposed to illness following repeated exposure to common psychosocial stressors. Elevated IL-6 has been implicated in the pathogenesis of coronary heart disease through a combination of autocrine, paracrine and endocrine mechanisms (Yudkin et al, 2000). A recent meta-analysis of studies measuring cytokine concentration in patients with major depression found IL-6 concentrations were significantly higher in depressed subjects compared with control subjects (Dowlati et al). The ability of proinflammatory cytokines to inhibit hippocampal neurogenesis was identified as one candidate mechanism for their detrimental effects on mood. Another mechanistic hypothesis, advanced to explain why children reared in unfavorable socioeconomic circumstances show increased susceptibility to chronic diseases of aging in adulthood, is that stress exposure in early life programs biological systems in a persistent and deleterious manner. Miller and colleagues performed genome-wide transcriptional profiling in healthy adults and found a history of socioeconomic stress during childhood was associated with up-regulation of genes bearing response elements for the CREB/ATF family of transcription factors that convey adrenergic signals to leukocytes (Miller et al, 2009). Additionally, they found subjects with high stress backgrounds had relative down-regulation of genes with response elements for the glucocorticoid receptor, increased output of cortisol in daily life, heightened expression of transcripts bearing response elements for NF-κB, and greater stimulated production of IL-6. Their results support the notion that exposure to socioeconomic adversity stress in early life leads to exaggerated adrenocortical and inflammatory responses via resistance to glucocorticoid signaling. While such a phenotype could be adaptive during acute threats to well-being, over time increasing allostatic load on the body might contribute to chronic disease processes. Cross-sectional measurements of HPA and immune function in adulthood may thus vary greatly as a function of relative age of an organism when stressed and/or the chronicity of exposure to a remote stressful environment. The relevance of timing and chronicity effects was illustrated by recent preclinical data demonstrating stress exposure in young rats resulted in the opposite direction changes in both central IL1-β and peripheral corticosterone concentrations than were observed when stress exposure was introduced in adulthood (Lu et al, 2010).
Consistent with this proposed mechanism, results from our past neuroendocrine investigations (Carpenter et al, 2007; Carpenter et al, 2009; Tyrka et al, 2008a; Tyrka et al, 2008b) as well as those reported by many other research groups (reviewed by Bauer et al, 2010; Gunnar et al, 2009; Heim et al, 2008) have associated early life stress with abnormal functioning of the hypothalamic-pituitary-adrenal (HPA) axis. We observed a pattern of relatively diminished cortisol response to the TSST among adults with histories childhood maltreatment (Carpenter et al, 2007), a subset of which comprise the sample for this current study of IL-6. However, we had not previously analyzed our cortisol data in relation to inflammatory cytokines or other peripheral markers of immune function. Investigation into complex relationships between IL-6 and cortisol in this small pilot study was limited by statistical considerations. However, we performed a cursory exploration with Pearson correlation coefficients generated for the individual time point data (plasma cortisol and IL-6 concentrations from the same stress test) in these 69 subjects. Consistent with patterns reported by others (Kunz-Ebrecht et al, 2003), we found cortisol concentrations to be inversely related to plasma IL-6 concentrations. The strongest and most statistically significant (negative) correlations emerged at the beginning and at the end of the 90-minute protocol, and were generally persistent after the application of CTQ total score as a control variable i.e., in a partial correlation analyses controlling for early life stress. It is possible that dampened cortisol response to acute stress in subjects with early life stress is partially responsible for the enhanced IL-6 stress response observed in this group (Heim et al, 2000) but more definitive studies will be needed to properly elucidate a causal relationship or how they interact to mediate the link between early adverse environment and adult health outcomes.
Measuring concentrations of other potential mediators, such as norepinephrine, in future studies of early life stress and inflammatory response to acute psychosocial stress will also be fruitful for parsing the complex interactions among the neuroendocrine, immune, and psychological systems (Bierhaus et al, 2003). An extensive literature documents a prominent role for sympathetic nervous system activation in both inflammatory and immunodepressive processes (Catania et al, 2009; Di Comite et al, 2007; Szelenyi and Vizi, 2007), and norepinephrine may offer some measure of neuroprotective effect by reducing brain infiltration of peripheral immune cells driven by increases in chemokine and cell adhesion molecule expression (O’Sullivan et al, 2010).
In conclusion, the results of this pilot study contribute to a rapidly growing literature which illuminates the complex relationships between stress and neuroimmune regulation in the pathoetiology of disease. Cytokines such as IL-6 are an integral part of the innate inflammatory response to a physical stressor (e.g. infection, inflammation). The mechanisms by which psychosocial stress initiates cytokine response, as well as the clinical consequences of an exaggerated cytokine response to stress, remain to be determined.
Acknowledgments
We thank Kelly Colombo, B.A., for her assistance with data management.
Footnotes
Disclosure/Conflict of Interest
This study was supported by R01 MH068767 (LLC). None of the authors has a competing interest with regard to this manuscript. The authors disclose the following biomedical financial interests and sources of support over the past three years and the foreseeable future. Drs. Tyrka, Price, and Carpenter have received grant/research support from the National Institutes of Health, NARSAD, Department of Defense, Sepracor, Cyberonics, Neuronetics, UCB Pharma, and Medtronic. Dr. Tyrka received honoraria for continuing medical education from Lundbeck and Takeda. Dr. Anderson has received grant/research support from the National Institutes of Health, the Korczak Foundation (Amsterdam) and the Alan B. Slifka Foundation (New York City). Dr. Price has received speakers’ bureau honoraria from Jazz Pharmaceuticals and MD Conferences/Psychiatry Review Course, consultant income from Abbott, Gerson Lehrman, Wiley, Qatar National Research Fund, Alberta Heritage Foundation for Medical Research, Springer, and Lundbeck. Dr. Carpenter has served as a consultant or on an advisory board for Abbott, AstraZeneca, Cyberonics, Novartis, Neuronetics, and Wyeth, and has received honoraria for continuing medical education from AstraZeneca and speakers’ bureau honoraria from Cyberonics and Neuronetics Ms. Gawuga received grant support from the National Institute of General Medical Sciences (R25 GM083270) to Brown University.
References
- Bauer ME, Wieck A, Lopes RP, Teixeira AL, Grassi-Oliveira R. Interplay between neuroimmunoendocrine systems during post-traumatic stress disorder: a minireview. Neuroimmunomodulation. 2010;17(3):192–195. doi: 10.1159/000258721. [DOI] [PubMed] [Google Scholar]
- Benson S, Arck PC, Tan S, Mann K, Hahn S, Janssen OE, et al. Effects of obesity on neuroendocrine, cardiovascular, and immune cell responses to acute psychosocial stress in premenopausal women. Psychoneuroendocrinology. 2009;34(2):181–189. doi: 10.1016/j.psyneuen.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100(4):1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton-Jarrett R, Fargnoli J, Suglia SF, Zuckerman B, Wright RJ. Association between maternal intimate partner violence and incident obesity in preschool-aged children: results from the fragile families and child well-being study. Arch Pediatr Adolesc Med. 2010;164(6):540–546. doi: 10.1001/archpediatrics.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Wright CE, O’Donnell K, Zachary I, Wardle J, Steptoe A. Stress-induced cytokine responses and central adiposity in young women. Int J Obes (Lond) 2008;32(3):443–450. doi: 10.1038/sj.ijo.0803767. [DOI] [PubMed] [Google Scholar]
- Butterweck V, Prinz S, Schwaninger M. The role of interleukin-6 in stress-induced hyperthermia and emotional behaviour in mice. Behav Brain Res. 2003;144(1–2):49–56. doi: 10.1016/s0166-4328(03)00059-7. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62(10):1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biol Psychiatry. 2009;66(1):69–75. doi: 10.1016/j.biopsych.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania A, Lonati C, Sordi A, Gatti S. Detrimental consequences of brain injury on peripheral cells. Brain Behav Immun. 2009;23(7):877–884. doi: 10.1016/j.bbi.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: the role of psychological stress. J Allergy Clin Immunol. 2006;117(5):1014–1020. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Chida Y, Sudo N, Sonoda J, Hiramoto T, Kubo C. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am J Respir Crit Care Med. 2007;175(4):316–322. doi: 10.1164/rccm.200607-898OC. [DOI] [PubMed] [Google Scholar]
- Cohen SKT, Mermelstein R. A Global Measure of Perceived Stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- D’Argenio A, Mazzi C, Pecchioli L, Di Lorenzo G, Siracusano A, Troisi A. Early trauma and adult obesity: is psychological dysfunction the mediating mechanism? Physiol Behav. 2009;98(5):543–546. doi: 10.1016/j.physbeh.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Comite G, Grazia Sabbadini M, Corti A, Rovere-Querini P, Manfredi AA. Conversation galante: how the immune and the neuroendocrine systems talk to each other. Autoimmun Rev. 2007;7(1):23–29. doi: 10.1016/j.autrev.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ. Neurohormonal-cytokine interactions: implications for inflammation, common human diseases and well-being. Neurochem Int. 2008;52(1–2):40–51. doi: 10.1016/j.neuint.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321–326. [PubMed] [Google Scholar]
- Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83(3):847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- Groer MW, Morgan K. Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology. 2007;32(2):133–139. doi: 10.1016/j.psyneuen.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology. 2009;34(1):62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26(5):447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- Keller P, Penkowa M, Keller C, Steensberg A, Fischer CP, Giralt M, et al. Interleukin-6 receptor expression in contracting human skeletal muscle: regulating role of IL-6. FASEB J. 2005;19(9):1181–1183. doi: 10.1096/fj.04-3278fje. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105(2):751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Mohamed-Ali V, Feldman PJ, Kirschbaum C, Steptoe A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain Behav Immun. 2003;17(5):373–383. doi: 10.1016/s0889-1591(03)00029-1. [DOI] [PubMed] [Google Scholar]
- LeMay LG, Vander AJ, Kluger MJ. The effects of psychological stress on plasma interleukin-6 activity in rats. Physiol Behav. 1990;47(5):957–961. doi: 10.1016/0031-9384(90)90024-x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Liu M, Shi S, Jiang H, Yang L, Liu X, et al. Effects of stress in early life on immune functions in rats with asthma and the effects of music therapy. J Asthma. 2010;47(5):526–531. doi: 10.3109/02770901003801964. [DOI] [PubMed] [Google Scholar]
- Merlot E, Moze E, Dantzer R, Neveu PJ. Cytokine production by spleen cells after social defeat in mice: activation of T cells and reduced inhibition by glucocorticoids. Stress. 2004;7(1):55–61. doi: 10.1080/1025389042000208150. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med. 2005;67(5):679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- O’Brien SM, Scott LV, Dinan TG. Cytokines: abnormalities in major depression and implications for pharmacological treatment. Hum Psychopharmacol. 2004;19(6):397–403. doi: 10.1002/hup.609. [DOI] [PubMed] [Google Scholar]
- O’Sullivan JB, Ryan KM, Harkin A, Connor TJ. Noradrenaline reuptake inhibitors inhibit expression of chemokines IP-10 and RANTES and cell adhesion molecules VCAM-1 and ICAM-1 in the CNS following a systemic inflammatory challenge. J Neuroimmunol. 2010;220(1–2):34–42. doi: 10.1016/j.jneuroim.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163(9):1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Powell ND, Bailey MT, Mays JW, Stiner-Jones LM, Hanke ML, Padgett DA, et al. Repeated social defeat activates dendritic cells and enhances Toll-like receptor dependent cytokine secretion. Brain Behav Immun. 2009;23(2):225–231. doi: 10.1016/j.bbi.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJGC, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychological Medicine. 1996;26(3):477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Spielberger C. Manual for the State-Trait Anxiety Inventory STAI (Form Y) 1983. [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21(7):901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Shintani F, Kanba S, Asai M, Nakaki T. Immobilization stress increases mRNA levels of interleukin-1 receptor antagonist in various rat brain regions. Cell Mol Neurobiol. 1997;17(5):557–562. doi: 10.1023/a:1026319107528. [DOI] [PubMed] [Google Scholar]
- Szelenyi J, Vizi ES. The catecholamine cytokine balance: interaction between the brain and the immune system. Ann N Y Acad Sci. 2007;1113:311–324. doi: 10.1196/annals.1391.026. [DOI] [PubMed] [Google Scholar]
- Threlkeld SW, Lynch JL, Lynch KM, Sadowska GB, Banks WA, Stonestreet BS. Ovine Proinflammatory Cytokines Cross the Murine Blood-Brain Barrier by a Common Saturable Transport Mechanism. Neuroimmunomodulation. 2010;17(6):405–410. doi: 10.1159/000288265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, et al. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008a;63(12):1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Wier L, Price LH, Ross NS, Carpenter LL. Childhood parental loss and adult psychopathology: effects of loss characteristics and contextual factors. Int J Psychiatry Med. 2008b;38(3):329–344. doi: 10.2190/PM.38.3.h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kanel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, et al. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41(9):744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Weinstein AA, Deuster PA, Francis JL, Bonsall RW, Tracy RP, Kop WJ. Neurohormonal and inflammatory hyper-responsiveness to acute mental stress in depression. Biol Psychol. 2010:228–234. doi: 10.1016/j.biopsycho.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148(2):209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- Zhou D, Kusnecov AW, Shurin MR, DePaoli M, Rabin BS. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology. 1993;133(6):2523–2530. doi: 10.1210/endo.133.6.8243274. [DOI] [PubMed] [Google Scholar]