Abstract
When drugs enter the brain rapidly liability for addiction is increased, but why this is the case is not well understood. Here we examined the influence of varying the speed of intravenous cocaine delivery on self-administration behavior in rats given limited or extended opportunity to take drug. The speed of cocaine delivery had no effect on self-administration behavior when rats were given only 1 hr each day to take cocaine. When given 6-fold more time to take cocaine rats that received cocaine rapidly (5 – 45 sec) increased their total intake 8-fold. However, rats that received cocaine more slowly (over 90 sec) did not avail themselves of the opportunity to take much more drug – they increased their intake only 2-fold. Furthermore, when tested 45 days after the last self-administration session a drug priming injection reinstated drug-seeking behavior only in rats that in the past had cocaine injected rapidly (5 sec), and this was associated with a persistent suppression in the ability of cocaine to induce immediate early gene expression. Cocaine may be potentially more addictive when it reaches the brain rapidly because (1) this promotes a marked escalation in intake and (2) it renders individuals more susceptible to relapse long after the discontinuation of drug use. This is presumably because the rapid uptake of drug to the brain preferentially promotes persistent changes in brain systems that regulate motivation for drug, and continuing exposure to large amounts of drug produces a vicious cycle of further maladaptive changes in brain and behavior.
Keywords: Drug Abuse, Nucleus Accumbens, Reinforcement, Extinction, Self-administration, transcription factor, Suppression
Introduction
Drugs or drug formulations that reach the brain rapidly are potentially more addictive than those that reach the brain relatively slowly (Hatsukami and Fischman, 1996). For example, people who initiate cocaine use through routes that lead to its rapid uptake into brain are more likely to become addicted (Gorelick, 1998; O'Brien and Anthony, 2005), but why this is the case is not well understood. One possibility is that cocaine’s euphorigenic effects are greatest when it is delivered rapidly (Resnick et al., 1977; Abreu et al., 2001; Nelson et al., 2006), which may enhance its reinforcing effects, promoting continued use (Gorelick, 1998). Alternatively, rapid drug delivery is more effective in producing forms of neurobehavioral plasticity that may promote the transition to addiction (Samaha et al., 2002). The rapid delivery of drugs like cocaine and nicotine greatly enhances their neurobiological effects (Brown and Kiyatkin, 2005; Samaha and Robinson, 2005), including their ability to induce immediate early genes in mesocorticolimbic structures (Samaha et al., 2004; Samaha et al., 2005), which is thought to be an initial step leading to forms of drug-induced plasticity important for addiction (Nestler, 2001; Zhang et al., 2005).
Despite the profound influence of rate of drug delivery on brain and behavior, evidence for a similar effect on the willingness of animals to self-administer drugs is mixed. In monkeys, increasing the rate of cocaine delivery facilitates self-administration behavior (Balster and Schuster, 1973; Kato et al., 1987; Panlilio et al., 1998; Woolverton and Wang, 2004), but in some studies such effects were evident only when the rate of infusion was so slow it would result in lower levels of drug in the brain (Balster and Schuster, 1973; Kato et al., 1987; Panlilio et al., 1998; Woolverton and Wang, 2004). In rats, when amphetamine or cocaine is delivered between 5 – 100 sec, a range that produces the same peak brain levels of drug but influences susceptibility to sensitization, there is very little, if any, effect on the acquisition or maintenance of self-administration behavior, progressive ratio performance, or the reinstatement of drug-seeking behavior (Pickens et al., 1969; Liu et al., 2005; Crombag et al., 2008). During acquisition rats do discriminate and preferentially select faster rates of cocaine within this range (Schindler et al., 2009), but it not clear whether this effect is sufficient to account for the influence of rate of drug delivery on susceptibility to addiction.
There are now a number of reports that rats develop addiction-like symptoms only when they are given extended access to cocaine (Ahmed and Koob, 1998; Ahmed et al., 2002; Deroche-Gamonet et al., 2004; Vanderschuren and Everitt, 2004; Pelloux et al., 2007; Ben-Shahar et al., 2008), but previous studies on rate of drug delivery employed limited access procedures (Pickens et al., 1969; Crombag et al., 2008). We hypothesized, therefore, that if given extended access to cocaine the degree to which rats would increase drug intake, and their later propensity for reinstatement, may vary depending on how rapidly cocaine was delivered.
Materials and Methods
Subjects and Housing
A total of 61 male Wistar rats (Harlan, IN) weighing between 225 – 250 g upon arrival were used. Rats were individually housed in a climate-controlled animal colony maintained on a 14:10 light/dark cycle (lights on at 0800), with food and water available ad libitum. All procedures were approved by the University of Michigan Committee on the Use and Care of Animals (UCUCA).
Rats were included in the experiment only if they acquired and maintained stable self-administration behavior and completed all phases of testing. A total of 46 rats completed the self-administration portion of the study. Rats were removed from the experiment mainly because they did not acquire self-administration or their catheters lost patency before completing the experiment. This did not impact one group more than the others. In the extinction-reinstatement portion of the experiment, one rat in the 90 sec group died during the course of the 30 day withdrawal period and was removed from this analysis.
Self-Administration Apparatus
Behavioral training and testing occurred in operant chambers located in a different room than the rat’s home cage. The chambers measured 22 × 18 × 13 cm (Med-Associates, VT), and were located inside larger sound-attenuating cabinets, which were continuously ventilated by a fan that also provided background noise. Each operant chamber was outfitted with two illuminated infrared nose-poke ports surrounding a centrally located pellet dispenser and pellet cup, and a red house light was positioned on the opposite wall. The floor of the chamber consisted of a grid floor with corncob bedding below the grids, which was replaced daily. Chambers were controlled by a PC computer (Intel Core Duo E4500) running Med-PC Version IV.
Each operant chamber was equipped with an infusion pump consisting of a 10 mL syringe installed in a PHM-100 pump (Med-Associates, VT) attached via a length of Tygon microbore tubing to an Instech-Solomon liquid swivel (Plymouth Meeting, PA) mounted on a counterbalanced arm. On training and test days rat’s catheters were attached to the liquid swivel via another length of PE tubing inserted within a flexible steel sheath. The rate of drug delivery was varied by installing motors (Model R-DE, Med-Associates, VT) capable of delivering a 50 µL infusion over 4.5 sec (2 RPM, 0.661 mL/min), 45.4 sec (0.2 RPM, 0.066 mL/min) or 90.8 sec (0.1 RPM, 0.033 mL/min). In referring to these groups, the rates are “rounded” and we will refer to groups receiving cocaine over 5, 45 or 90 sec. Prior to each use, the apparatus was flushed with a 20% ethanol aqueous solution (V/V) to ensure adequate liquid flow through the swivel and lines. Additionally, every 20 days the infusion efflux delivered by the apparatus was volumetrically measured.
Pretraining
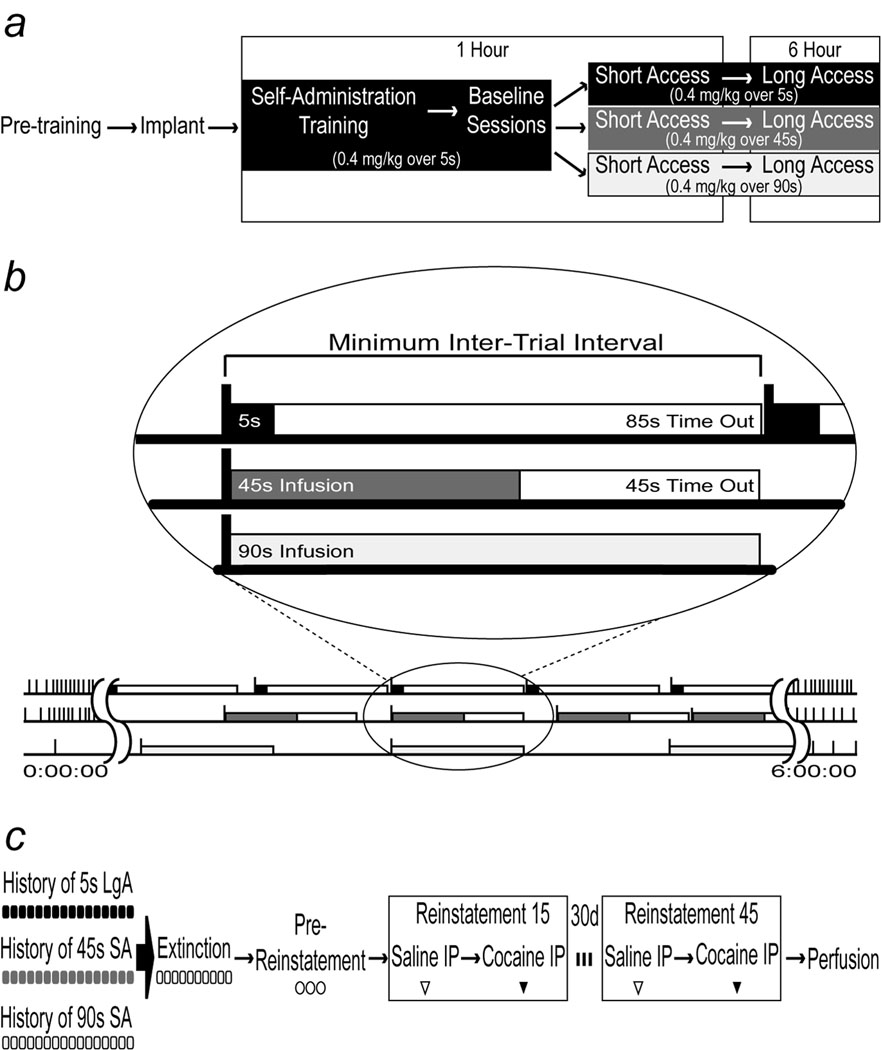
An illustration of the general self-administration experimental design is shown in Fig. 1a. To facilitate the acquisition of cocaine self-administration, rats underwent operant training using a food reward prior to jugular catheterization. On the first day, rats were habituated to the operant chamber, and one food pellet (45 mg Banana flavored Dustless Precision Pellets, Bio-Serv, NJ) was delivered into the pellet cup at a pseudo-random interval approximately every 30 sec during a 30 min session. On the next three days, rats were trained to nosepoke for food reward using a continuous schedule of reinforcement (FR1). At the start of each session, the house light was turned on and the “active” nosepoke port was illuminated for 20 sec. Responses in the active port resulted in the delivery of one pellet. Responses in the “inactive” port did not have any programmed consequences. Rats were then transitioned to a fixed ratio 2 (FR2) schedule for a minimum of three days. We used food pretraining because during cocaine self-administration no explicit cue was associated with drug delivery (see below), which retards the acquisition of this behavior, and so we used food pretraining to help ameliorate this situation.
Fig. 1.
Panel a: An illustration of the self-administration experimental design. Panel b: a representation of a typical long access session for rats in the 5, 45 and 90 sec groups. In the bottom portion of the figure, the length of the session is represented by a horizontal line. Vertical tick marks represent nosepokes where rats received an infusion of cocaine. For clearer comparison, the inset circle shows the infusion lengths and timeout intervals of the three groups in the experiment. The duration of the infusion and following timeout interval is indicated for each group. Note that the number of opportunities to self-administer an injection is exactly the same in all groups because the timeout periods varied accordingly. Panel c: an illustration of the extinction-reinstatement experimental design. Icons depict the number of sessions for each condition.
Catheter Construction and Surgical Procedures
Chronically indwelling jugular catheters with a dead volume of 24–26 µL were placed into rats using a procedure similar to that described elsewhere (Weeks and Davis, 1964; Weeks, 1972; Crombag et al., 1996; Samaha et al., 2002). While not in use, catheters were capped with obdurators. Briefly, rats were anesthetized with ketamine/xylazine (100 mg/kg and 10 mg/kg, intraperitoneally, i.p.). The backport was secured between the rat’s shoulder blades, while the silicon end of the catheter was inserted into the left or right external jugular vein. Catheters were then filled with 0.1 mL solution of 10 mg/mL gentamicin sulfate (Vedco, St. Joseph, MO), in 0.9% sterile bacteriostatic saline to minimize infections and catheter occlusions.
Rats were allowed to recover for a minimum of 4 days. Starting the day after the surgery catheters were flushed manually once per day with 0.2 mL of the gentamicin solution. Every 7 days rats were infused intravenously with 0.2 mL sodium thiopental solution (20 mg/mL, dissolved in sterile water, Hospira, Lake Forest, IL) to assess catheter patency. Rats that did not become ataxic within 5–10 sec were removed from the study. Rats did not undergo self-administration training on their catheter patency test days.
Drugs and self-administration training
Cocaine hydrochloride (National Institutes of Drug Abuse, MD) was prepared in a 0.9% sterile saline solution. During self-administration sessions rats received infusions consisting of 0.4 mg/kg cocaine (weight of the salt) in a volume of 50 µL. The unit dose was adjusted every four days.
After recovery from surgery, rats were re-acclimatized to the operant chamber by an additional session with an FR2 response requirement for a food pellet. The following day all rats began daily cocaine self-administration training sessions. All training sessions were one hour long, and in all training sessions all rats received cocaine delivered over 5 sec. Before each session, catheters were flushed with 0.2 mL of the gentamicin solution, and then attached to the infusion apparatus. Upon an experimenter-initiated command, the infusion pumps were activated to fill the dead volume of the catheters prior to the start of session. Once catheters were filled, the session began with the illumination of the house light and a 20 sec illumination of the active nosepoke port. Initially, rats were trained on a continuous reinforcement schedule (FR1) receiving cocaine followed by an unsignaled 20 sec timeout interval, where further responses were recorded but had no consequences. Note, therefore, that there was no cue explicitly paired with drug delivery, because this would have introduced a confound when the rate of drug delivery was subsequently varied (see below). Once rats were responding above a minimum training criterion (2 or more injections per session, twice as many active versus inactive nosepokes) for two consecutive days, the response requirement was increased to an FR2 schedule of reinforcement. After achieving stable performance above the minimum training criterion at this schedule for two consecutive days, the timeout interval in subsequent daily sessions was lengthened from 20 sec to 45 sec, then 63 sec, and finally 85 sec. The reason for increasing the final timeout period to 85 sec was so that in subsequent sessions using different rates of infusion the total number of infusions possible for all subjects in all groups would remain the same. That is, regardless of infusion rate all rats could only take one injection every 90 sec (see Fig. 1b).
Influence of Rate of Infusion: Short Access (1 hr) Sessions
After acquiring cocaine self-administration on a FR2 schedule of reinforcement during one hour sessions where cocaine was injected over 5 sec with an 85 sec timeout interval (this is referred to as “Baseline”), rats were assigned to one of three groups that differed in rate of cocaine delivery (Fig. 1a, b). One group (“5 sec”, n=14) continued to receive cocaine infusions at the training rate of infusion and with the same timeout interval. A second group (n=18) received their infusions over 45 sec followed by a 45 sec timeout interval. The last group (n=14) was infused with cocaine delivered over 90 sec, with no timeout interval (Fig. 1b). Individual subjects were assigned in such a way that the mean number of infusions achieved during the last two days of baseline training was balanced between groups. Rats were tested for three daily sessions at their respective new rates during 1 hr short access (ShA) sessions.
Influence of Rate of Infusion: Long Access (6 hr) Sessions
From Day 4 to Day 20 of self-administration testing the length of each session for all groups was increased to six hrs/day, as this procedure is reported to lead to the development of addiction-like symptoms (Ahmed and Koob, 1998, 1999; Ahmed et al., 2002). Once the sessions were extended they all began at 1100 hr in order to reduce the influence of circadian rhythms on self-administration performance. Rats were removed from the study during long access (LgA) sessions if they failed to maintain a median number of infusions greater than 2.5 and a median active/inactive nosepoke ratio greater than 1 throughout the experiment.
Extinction Training
An illustration of the general design of the extinction and reinstatement experiment is shown in Fig. 1c. After their last LgA self-administration session rats underwent 10 days of extinction training, using procedures similar to those described previously (De Vries et al., 1998; Shalev et al., 2002; Crombag et al., 2008). During these sessions, rats were taken from the animal colony, placed into test chambers and attached to the infusion apparatus. The session proceeded in an identical fashion to self-administration sessions, except that a nosepoke into the active port activated the infusion pump without delivery of drug. Each extinction session was one hour in length. Following extinction sessions, rats underwent three 1 hr pre-reinstatement sessions where the rats were not connected to the infusion apparatus, and the operant chamber remained unlit for the entire session. Responses to either nosepoke port were recorded but had no other consequences. These sessions were conducted to further reduce the contribution of possible conditioned stimuli associated with drug delivery on responding (De Vries et al., 1998), and reduce possible contributions of novelty introduced by the operational changes between extinction sessions and reinstatement sessions.
Reinstatement Testing
The following day (14 days after the last self-administration session), rats were injected with saline i.p. and immediately placed in the self-administration chamber for 2 hrs. Similar to pre-reinstatement sessions, stimuli associated with drug delivery such as the attachment to the infusion line, house light, and infusion pump that might confound or potentiate reinstatement of drug-seeking behavior were not present for the duration of the session. Responses to the previously active and inactive nose-pokes were recorded, but had no consequence. The next day rats were tested for the reinstatement of drug-seeking behavior by giving them a 10 mg/kg i.p. injection of cocaine prior to placement into the test chamber. Thus, the first reinstatement test took place 15 days after the last self-administration session. Active and inactive nosepokes were recorded, although they had no consequence. The rats were then left undisturbed for an additional 30 days, after which time the cocaine priming reinstatement test was repeated as described above. Thus, the second test for reinstatement took place 45 days after the last self-administration session.
Fos Immunohistochemistry
A subset of rats with self-administration and reinstatement experience were used for Fos immunochemistry (5 sec, n=6; 45 sec, n=10; 90 sec, n=8), and an additional 15 drug naïve control rats were used as controls for this portion of the study. These control rats were moved daily from the home colony room to holding chambers concurrently with rats undergoing self-administration training, where they were left undisturbed for 6 hrs a day for an average of 10 days. Control rats were matched with rats or groups of rats undergoing their final reinstatement test. During these days, control rats spent two hours in their transport chambers, mirroring the length of the reinstatement session. All control rats received saline injections on the same day as rats undergoing reinstatement testing. The following day, some control rats received a second saline i.p. injection (n=5), while others (n=10) received an acute 10 mg/kg i.p. injection of cocaine. In this way, these control rats experienced the general effects of handing, transport, and time outside the animal colony in a distinctly different environment similar to self-administration rats (Ferrario et al., 2005). The control rats remained either drug naïve or experienced a single acute exposure to cocaine equivalent to the dose and route of administration as the cocaine priming injection used to reinstate cocaine seeking behavior in rats that had undergone extinction training.
Immediately after the end of the second drug-primed reinstatement session or 2 hours after injecting a transport control, rats were taken to a different room and deeply anesthetized with sodium pentobarbital (390 mg/kg i.p., Vortech, Dearborn MI), and transcardially perfused with 500 mL ice cold perfusion rinse (73 mM sucrose, 18 mM procaine hydrochloride, 139 mM sodium chloride in 0.1 M sodium phosphate buffer (SPB), pH 7.4) followed by 250 mL ice cold paraformaldehyde rinse (4% (W/V) paraformaldehyde dissolved in 0.1 M SPB buffer containing 73 mM sucrose, pH 7.4). Brains were removed and placed in 4% formaldehyde overnight at 4 degrees Celsius, and then transferred to 30% (W/V) sucrose for 3 days at the same temperature. Brains were coronally sectioned to a 40 µm thickness with a freezing microtome. Series of sections were collected 120 or 160 µm apart. Sections that were processed for immunohistochemistry within 2 days were stored in 0.1 M SPB, otherwise sections were stored at −20 Celsius in liquid cryoprotectant. Sections were processed for Fos and Mu opioid receptor protein immunofluorescence in a similar manner to Reynolds and Berridge (2008). Mu opioid receptor expression was used to delineate the boundaries of the subregions of the nucleus accumbens (Heimer et al., 1997). Sections were washed in 0.1 M SPB containing 0.2% (V/V) Triton-X with gentle agitation for 30 minutes, and then pre-blocked for 30 minutes in a 5% (V/V) Normal Donkey Serum (NDS) solution. Sections were then incubated overnight with a primary antibody solution containing 1:500 goat polyclonal antibody raised against the N-terminus of human Fos (sc-52-G, Santa Cruz, Santa Cruz, CA) and 1:1000 rabbit polyclonal antibody raised against the C-terminus of the rat Mu opioid receptor 1 (ab10275, Abcam, Cambridge, MA). After washing in 0.1 M SPB for 30 minutes, sections were pre-blocked in a solution containing 5% NDS and 5% (V/V) Image-iT FX signal enhancer (Invitrogen, Eugene, OR) for 30 minutes. Sections were subsequently incubated for 2 hours in a solution containing 1:250 donkey anti-goat AlexaFlor 488 antibody and 1:250 donkey anti-rabbit AlexaFlor 594 antibody (Invitrogen, Carlsbad, CA) and 5% signal enhancer. Next, sections were washed in SPB buffer and mounted, air-dried, and coverslipped with Prolong Gold anti-fade reagent (Invitrogen, Eugene, OR). Control tissue stained with only secondary antibodies revealed little or no unspecific binding.
Visualization
Sections were visualized at 100× total magnification with a Leica DM6000B (Wetzlar, Germany) microscope with a Leica EL6000 external fluorescent light source coupled to a Q-Imaging monochrome 12 bit 1.4 megapixel digital camera (Surrey, Canada). AlexaFlor 488 was fluoresced using a Leica L5 bandpass filter cube, and AlexaFlor 594 was fluoresced using a TX2 bandpass filter cube. The same section and region was imaged for Fos and Mu opioid immunoreactivity. Separate Fos and Mu opioid images were captured as LZW lossless compression TIFF files to minimize image compression artifacts in dark-field areas of the image and were analyzed using the MCID Core 7.0/Analysis (Cambridge, UK) software package.
Sampling Areas
The number of Fos positive cells in the core and shell of the nucleus accumbens from the left and right hemispheres was summed together. The nucleus accumbens (NAc) shell and core were sampled at +2.0 mm from bregma (Paxinos and Watson, 1998). An elliptical sampling area of 300 µm × 650 µm was used to sample the shell, and a smaller sampling area of 300 µm × 450 µm was used for the core. To better follow the contours of the shell and the core, sampling ellipses were angled.
Quantification
The number of Fos positive cells was quantified by using the “Autoscan Utility”, where a maximum of 5 Fos positive cells were visually identified in each image by the experimenter and sampled for hue and intensity values using a 4 micron diameter sampling circle. The average value provided the threshold for identifying and quantifying Fos positive nuclei in the image. In addition, a “Target selection criteria” optimized to reduce the incidence of image artifacts contributing to the final count was used. Targets thus identified by the “Autoscan Utility” were then overlaid on the original unaltered Fos image and visually inspected to ensure fidelity in quantification and to minimize the contribution of artifacts towards the final cell count. In cases where no Fos positive nuclei could be identified in the section, Fos-positive nuclei from the cortex immediately adjacent to the striatum were used to set the target threshold. In all cases the person quantifying the images was blind to the experimental conditions of each image.
Statistics
Behavioral data during LgA training sessions was analyzed as bins comprised of the average of two contiguous daily sessions, as this reduced day to day variation and simplified data presentation. Data with repeated measures over sessions or time were analyzed using Linear Mixed Models analysis (LMM) using the SPSS 16 statistical package. For LMM analysis, the best fitting model of repeated measures covariance was determined by the lowest Akaike Information Criterion (AIC) score (West, 2007). Depending on the model selected, the degrees of freedom may have been adjusted to a non-integer value. If the LMM resulted in significance, mixed model pairwise multiple comparisons of repeated measures means (Kowalchuk and Keselman, 2001) were used to test for group differences.
Behavioral and Fos immunohistochemistry analysis involving between group comparisons were analyzed using one-way analyses of variance (ANOVA) or two-way ANOVA followed by a Fisher’s LSD post-hoc test or one tailed t-tests, where appropriate.
Results
Training
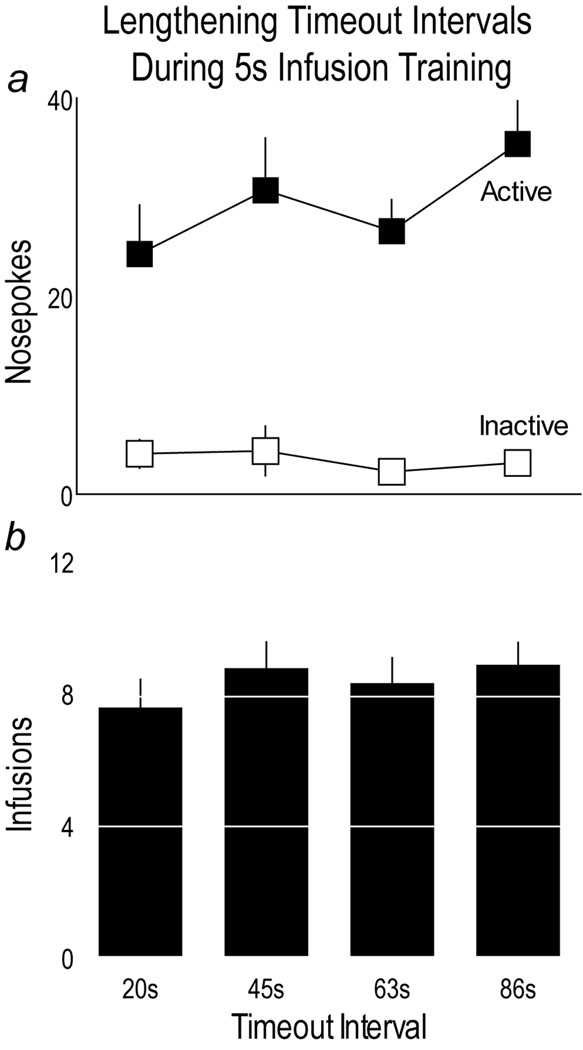
Fig. 2 shows the influence of gradually increasing the timeout interval during training on the number of active and inactive nosepokes (Panel a) and cocaine infusions (Panel b). During these training sessions, prior to being assigned to groups, all rats self-administered 0.4 mg/kg/inf cocaine delivered over 5 sec. There was no effect of progressively increasing the timeout interval on either active nose pokes (p>0.05) or the number of infusions (p>0.05).
Fig 2.
Panel a: The mean (±SEM) number of active and inactive nosepokes made by all rats during self-administration training sessions prior to group assignment, where timeout intervals were gradually lengthened. Panel b: The mean number of infusions made by all subjects during self-administration training sessions. During these training sessions all rats received the cocaine infusion at the same speed (over 5 sec), and the timeout intervals were increased each time a rat reached criterion performance for self-administration. Data presented in the 85 sec timeout sessions in this figure is the average of all subjects comprising the “Baseline” data point presented in Fig. 3.
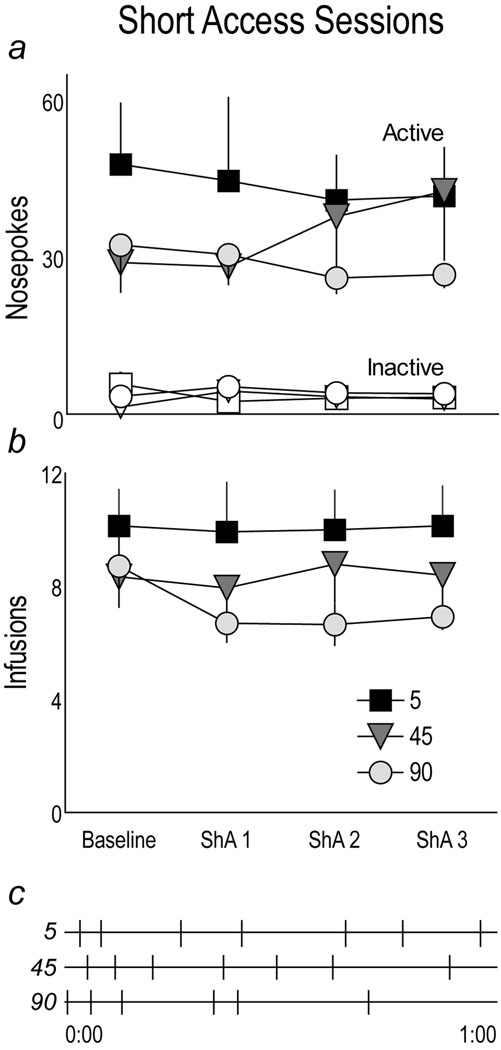
In Fig. 3a “Baseline” indicates the mean number of active and inactive nosepokes (Panel a) and cocaine infusions (Panel b) averaged over the last two training sessions when all rats received cocaine over 5 sec and all had a 85 sec timeout interval (these 3 groups represent rats that on the next day will receive cocaine at different rates). There was no effect of group (p>0.05) or group by port interaction (p>0.05) for nosepokes, although all groups made more active than inactive responses (F(1,84)=54.68 p<0.0001). There were also no significant group differences in the number of infusions during “Baseline”. (Fig. 3b; p>0.05)
Fig. 3.
Panel a: The mean (±SEM) number of active and inactive nosepokes made during baseline training sessions, when all rats received cocaine at the same rate (5 sec), and during short access (ShA) sessions when rats were assigned to groups that received cocaine over either 5, 45, or 90 sec. Baseline data are averaged over 2 sessions. Panel b: The mean (± SEM) number of infusions received during the same baseline training and ShA sessions as Panel a (note Log2 scale). Panel c: the pattern of self-administration behavior in 3 representative rats during ShA session 3. The horizontal line represents the duration of the session, and vertical marks indicate the time during the session when a rat injected itself with cocaine.
Short Access Sessions
Fig. 3a also shows the number of nosepokes made during three daily ShA sessions (ShA1–3) after rats were assigned to groups that received cocaine over 5, 45 or 90 sec. There was no significant effect of group, session, or a significant interaction (all p’s>0.05). Fig. 3b shows that for the number of infusions there were also no effects of group, session, or a group by session interaction (all p’s>0.05). Fig. 3c shows the number of infusions achieved by three representative subjects from each group during the last short access session.
In summary, during ShA sessions there was no significant effect of varying the rate of cocaine delivery between 5 and 90 sec on self-administration behavior, as assessed by either the number of active nosepokes or total infusions received during the session. This is consistent with our more extensive previous studies on the effect of rate of cocaine or amphetamine delivery on self-administration behavior under limited access conditions (Crombag et al., 2008).
Long Access Sessions
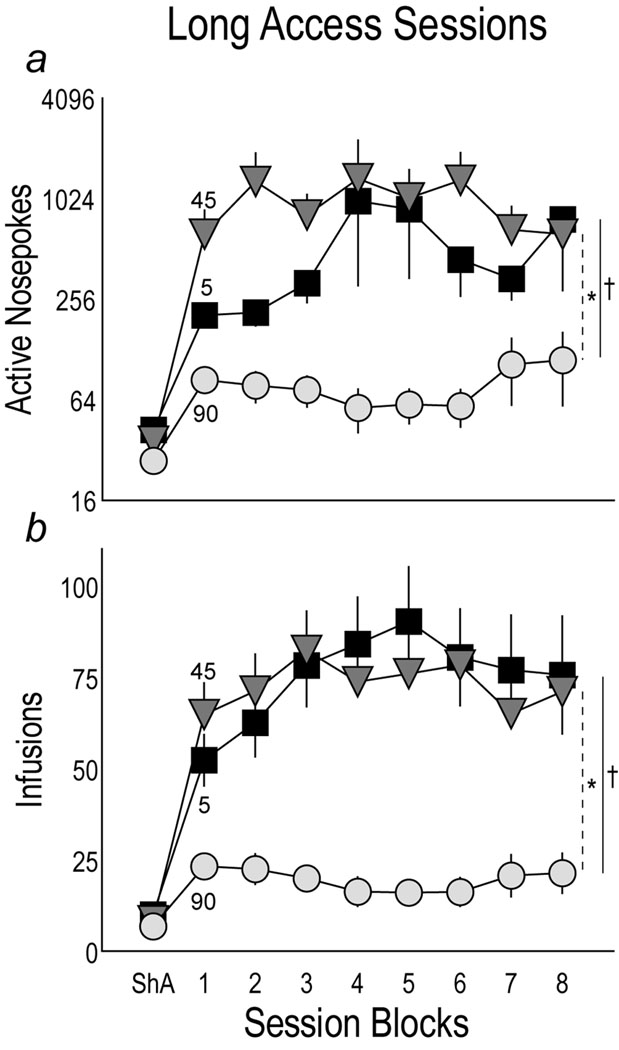
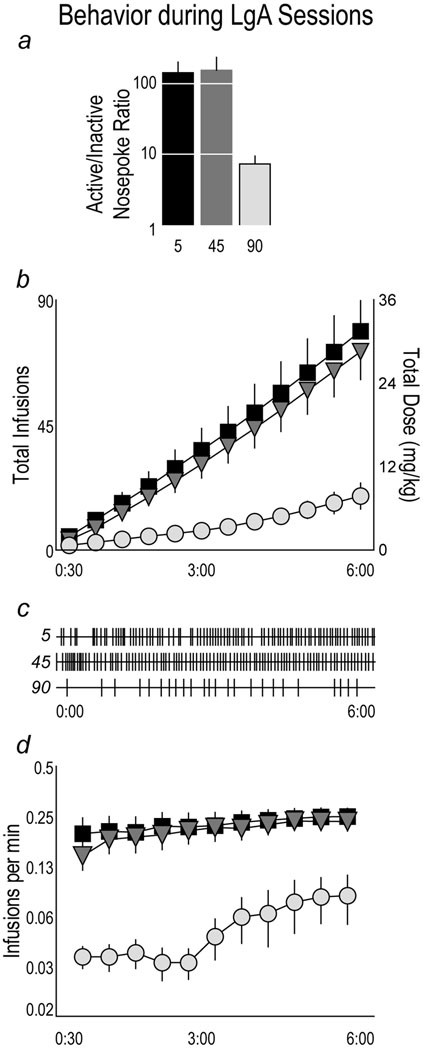
Fig. 4 shows the mean number of active nosepokes (Panel a) and cocaine infusions (Panel b) made by rats in each group when the session length was extended from 1 hr/day (ShA) to 6 hours/day (Long Access, LgA). LgA sessions were analyzed as blocks of two daily sessions, and the average for the three ShA sessions is shown for comparison. Not surprisingly, all groups increased both their total number of nosepokes and infusions when the test session was extended from 1 to 6 hrs/days. To determine whether active nosepokes differed by group during LgA sessions, an overall LMM analysis with session blocks as a repeated measure was conducted on the number of active nosepokes during LgA sessions. There was an overall effect of group (F(2,43)=3.562 p<0.038), but no effect of session (p>0.05), and no interaction (p>0.05). To determine how the groups differed from one another during LgA sessions, each group was compared using paired repeated effects LMM analysis. The 5 sec and 45 sec groups made significantly more nosepokes than the 90 sec group, but did not differ from one another, although there was a trend for rats in the 45 sec group to make more unrewarded responses during the timeout period than rats in the 5 sec group.
Fig. 4.
shows the mean (± SEM) number of active nosepokes (Panel a; note Log10 scale) and cocaine infusions (Panel b) made by rats receiving 0.4 mg/kg/inf cocaine over 5, 45 or 90 sec after self-administration sessions were lengthened from 1 hour to 6 hours/day (“long access”, LgA). LgA session data were analyzed as blocks of two daily sessions, and the average for the ShA sessions is shown for comparison. In Panel a, (†) indicates 5 vs. 90 sec, p<0.011, and (*) indicates 45 vs. 90 sec, p<0.024. In Panel b, (†) indicates 5 vs. 90 sec, p<0.001 and interaction p<0.004. (*) indicates 45 vs. 90 sec, p<0.001.
For infusions (Panel b) there was an overall effect of group (F(2,45)=13.986, p<0.001), but no effect of session (p>0.05) and no group by session interaction (p>0.05). The 5 sec and 45 sec groups took significantly more infusions than the 90 sec group, but did not differ from one another (p>0.05).
In summary, Fig. 4 shows that when given more time to take cocaine rats given injections over 5 or 45 sec markedly increased their number of nosepokes and associated cocaine intake, and did so to a comparable extent. In striking contrast, rats given cocaine over 90 sec only modestly increased their drug intake relative to what they took when drug was only available for 1 hr/day. Indeed, rats in the 5 and 45 sec groups increased their cocaine intake about 8-fold when given a longer time to self-administer, but despite 6-fold more time available to take cocaine, rats in the 90 sec group only increased their intake about 2-fold.
Within Session Pattern of Self-Administration
Fig. 5a shows that during the LgA sessions all rats in all groups discriminated between the active and inactive nosepoke ports. Fig. 5 also illustrates the temporal pattern of self-administration behavior within LgA sessions. Fig. 5b shows that the 5 and 45 sec groups maintained a stable and high rate of self-administration throughout the duration of LgA sessions. The cumulative infusions in the 90 sec group was much lower than in the other groups, but did progressively increase as the session progressed (effect of session bin, F(11,46)=8.822 (p<0.001), indicating that rats in the 90 sec group continued to take cocaine throughout the session. This is also illustrated in Fig. 5d, which shows representative data from individual subjects (the same rats as in Fig. 3c). Finally, the rate of self-administration (infusions/min) within sessions is shown in Fig. 5d, confirming that the 5 and 45 sec groups maintained a stable rate of self-administration for the duration of the session. In the 90 sec group the rate was even somewhat elevated in the last 3 hrs relative to the first 3 hrs of the session (effect of session bin, F(10,13)=4.365 p<0.009). This is consistent with the small upward deflection seen in cumulative infusions during the latter half of the session. Thus, all groups continued to self-administer cocaine throughout the duration of the 6 hr sessions.
Fig. 5.
Panel a: The mean (+SEM) ratio of active to inactive nosepokes made by groups during long access sessions. All groups discriminated between the active and inactive ports. Panels b–d show how self-administration behavior was distributed within the last 6 LgA sessions. Panel b: Mean (±SEM) cumulative infusions and total dose of cocaine self-administered by rats receiving cocaine over 5, 45, 90 sec during 6 hr LgA sessions. Panel c: Infusions during LgA session 14 in the same 3 representative rats as shown in Fig 3.3c. Panel d: The mean (±SEM) rate of self-administration (infusions/min) within LgA sessions.
Analysis of the First Hour of the LgA Sessions
It has been reported that when given extended access to cocaine rats increase not only their total intake, but over time they escalate the amount of drug they take during the first hour of each session (Ahmed and Koob, 1998, 1999; Ahmed et al., 2002). When all subjects were included in an analysis, rats in neither the 5 sec nor 45 sec groups significantly escalated their number of infusions during the first hour of LgA sessions, as compared to ShA sessions (both p’s>0.05), and rats in 90 sec group actually decreased infusions in the first hour over days of testing (F(8,104)=10.429 p<0.001).
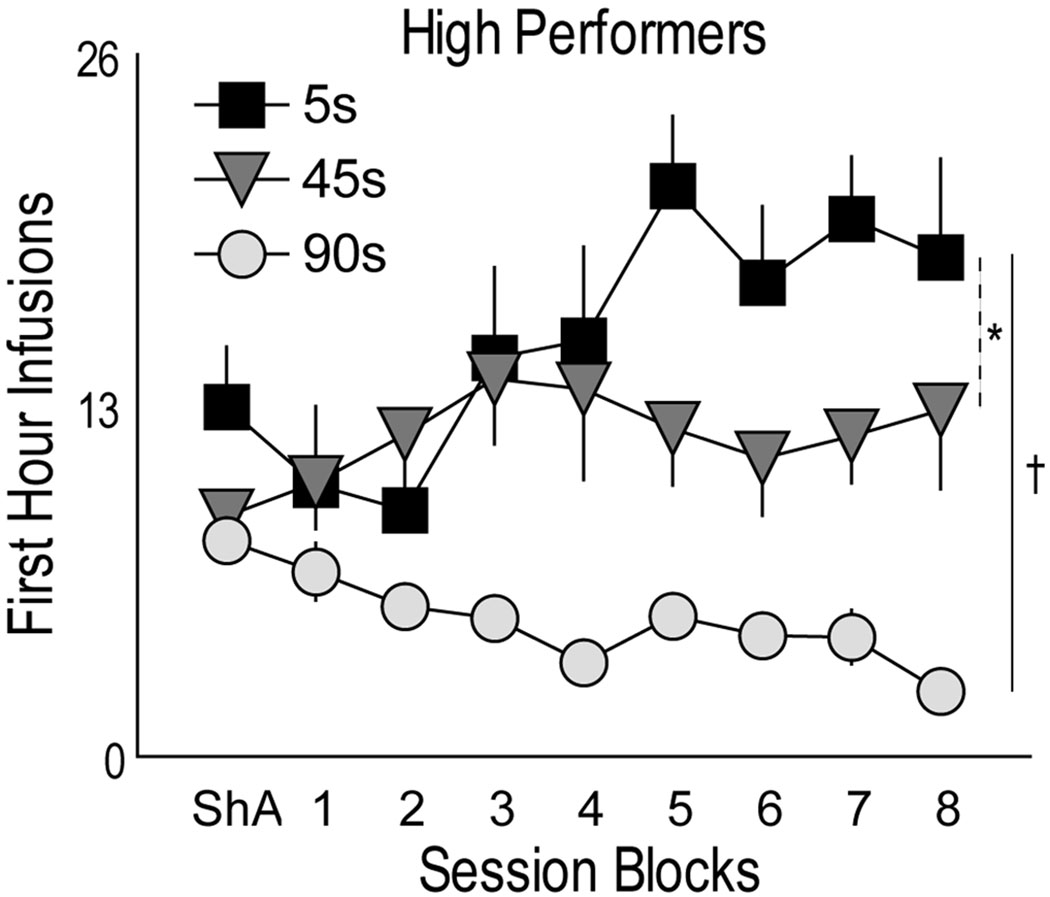
However, there was considerable individual variation in first hour intake. Therefore, rats within each group were divided into “high performers” (top 50%) and “low performers” (bottom 50%), based on the total number of first hour infusions during the last 8 days of LgA testing. The low performers in all groups showed a small decline in first hour infusions across days of LgA testing (data not shown). In contrast, “high performers” (Fig. 6) in the 5 sec group tended to increase their first hour intake across days of testing (F(8,37)=2.122 p=0.058), whereas rats in the 45 sec group showed no change across sessions (p>0.05). The difference between the 5 and 45 sec groups was statistically significant. This analysis suggests, therefore, that a subset of rats (“high performers”) given a 5 sec infusion of cocaine escalated their first hour cocaine intake to a greater extent than rats given cocaine over 45 or 90 sec.
Fig. 6.
The mean (±SEM) number of infusions during the first hour of each session in “high performers” in each group, plotted over days of testing. “High performers” in each group were those that took above the median number of injections (top 50%) during the first hour (averaged across all LgA sessions). (*) indicates 5 vs. 45 sec, p<0.05, and (†) indicates 5 vs. 90 sec, p<0.001 and interaction p<0.045.
Extinction
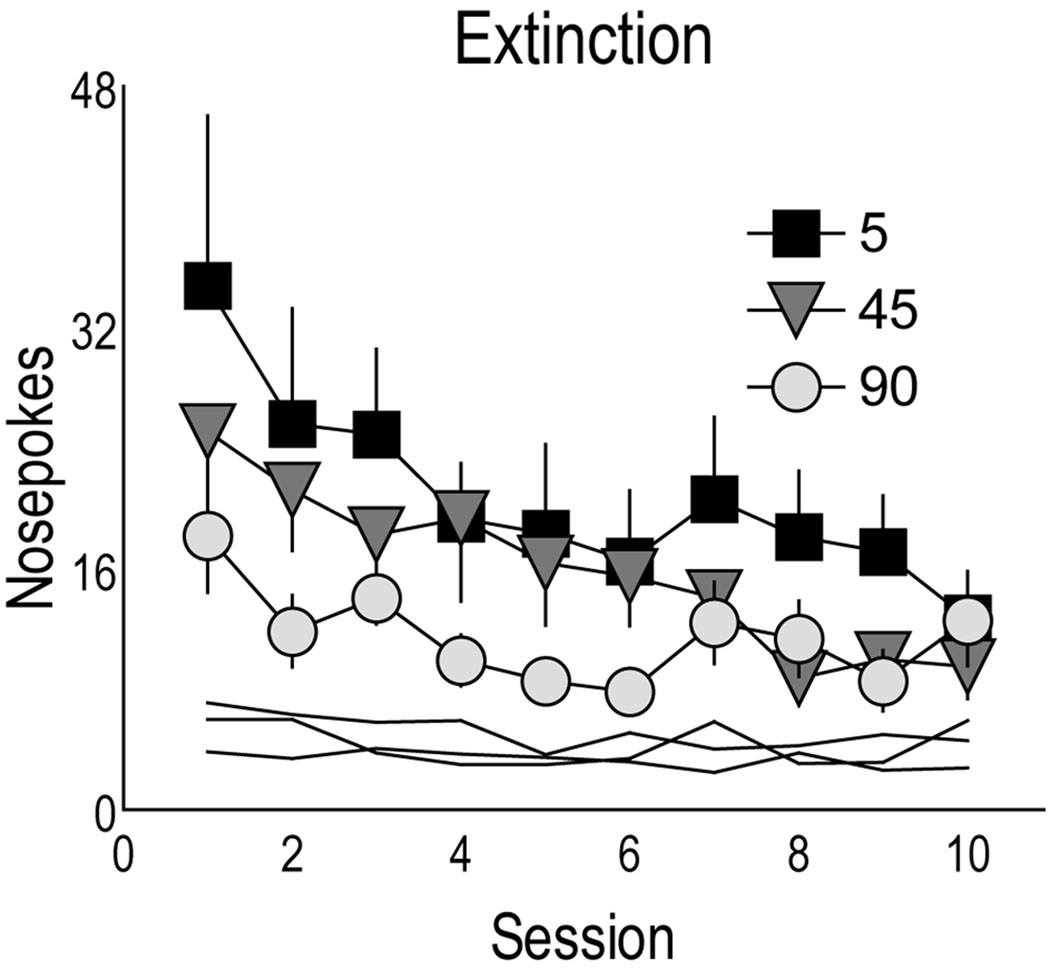
Fig. 7 shows the number of active and inactive nosepokes by rats in each group during ten sessions of extinction training. Active nosepokes are illustrated by symbols, while inactive nosepokes are shown as lines. There was a comparable decrease in active nosepokes over time in all groups (effect of session F(9,43)=2.584 p<0.019). Although it appears that rats in the 5 sec group made more responses during extinction than those in the 90 sec group, this was not statistically significant (effect of group and interaction, p>0.05).
Fig. 7.
The mean (±SEM) number of nosepokes made by rats in each group during 10 days of extinction training when cocaine reward was not available (note Log2 scale). Active nosepokes are shown using symbols and inactive nosepokes are depicted as lines.
First Reinstatement Test (15 days after the last self-administration session)
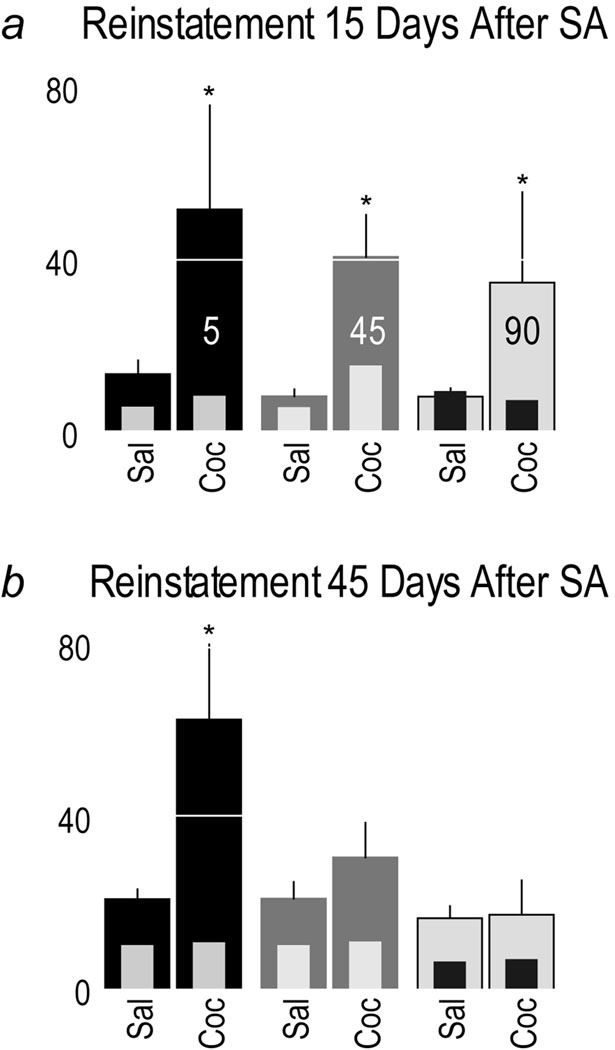
Fig. 8 (Panel a) shows the number of nosepokes after an i.p. priming injection of either saline or cocaine given 15 days after the last self-administration session and extinction training. Active nosepokes are represented by large bars and inactive nosepokes are represented by inset bars. Rats in all groups reinstated responding following a cocaine priming injection, as indicated by a significant increase in the number of active nosepokes, and there were no significant group differences in the degree of reinstatement (main effect of treatment F(1,84)=9.403 p<0.004; effect of rate of infusion (ROI) history, and treatment × ROI history interaction, p’s>0.05). There were no group differences in inactive nosepokes (p’s>0.05).
Fig. 8.
Panel a shows the mean (+SEM) number of nosepokes during the first reinstatement test, conducted 14–15 days after the last self-administration session, and 3 days after extinction training was completed. On Day 14 all rats received an injection of saline i.p. (Sal) and the next day (Day 15) an injection of cocaine i.p. (Coc). (*) indicates a main effect of cocaine treatment (p<0.004). Panel b shows data from the second reinstatement test, which was conducted 45 days after the last self-administration session and 30 days after the first reinstatement test. The number of active nosepokes are represented by thick bars, and inactive nosepokes by thin inset bars. (*) indicates saline vs. cocaine in the 5 sec group t(13)=2.383 p<0.0167.
Second Reinstatement Test (45 days after the last self-administration session)
Fig. 8 (Panel b) shows the number of nosepokes during the test for reinstatement conducted 45 days after the last self-administration session, and 30 days after the first reinstatement session. For active nosepokes a two-way ANOVA resulted in a significant main effect of treatment (F(1,84)=6.081 p<0.017) and of ROI history (F(2,84)=3.991 p<0.023), and the treatment by ROI history interaction was almost significant (F(2.84)=2.967 p=0.057). To determine which groups reinstated responding active nosepokes following the cocaine prime were compared with those produced by an injection of saline. Only rats that previously received cocaine over 5 sec showed significant reinstatement (t(13)=2.383 p<0.0167).
Similar to the first reinstatement test, there were no group differences in the number of inactive nosepokes. Note, however, that in all groups the number of active nosepokes following the Saline injection were greater in this test than the first test for reinstatement (compare panels a and b), suggesting modest spontaneous recovery of responding.
Fos Immunoreactivity After Cocaine Priming
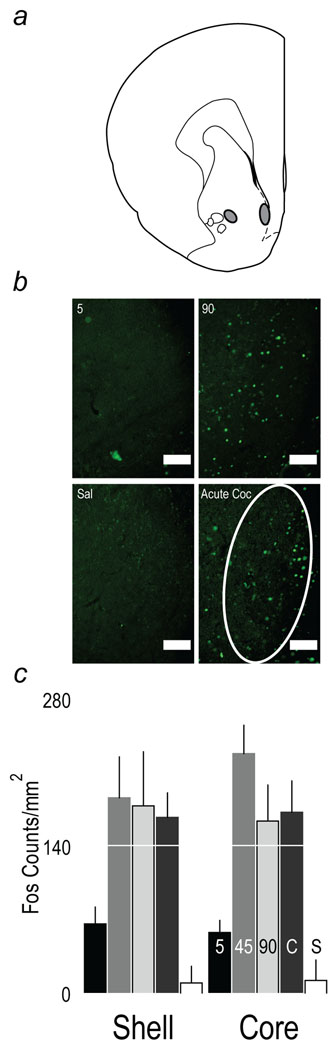
Areas sampled are indicated by the grey areas in Fig. 9a. Representative images showing Fos positive cells and areas sampled within the nucleus accumbens core and shell are shown in Fig 9b. Fig. 9c illustrates the number of Fos positive nuclei per mm2 in the core and shell of the nucleus accumbens two hours after an i.p. priming injection of cocaine on the second reinstatement test day.
Fig. 9.
Panel a: areas of the core and shell divisions of the nucleus accumbens +2.0 mm from bregma marked in gray that were sampled at the end of the reinstatement session 2 hours after an i.p. injection of 10 mg/kg of cocaine. Panel b: examples of Fos positive nuclei in the shell of the nucleus accumbens +2.0 from bregma (Fos-positive nuclei are labeled green). At the start of the second reinstatement session rats were given an i.p injection of saline or 10 mg/kg of cocaine and brains obtained 2 hours later. The panels are from rats that had a history of cocaine self-administration, with cocaine delivered over 5 sec or 90 sec, and controls that received an i.p. injection of saline (Sal) or an acute (first time) injection of cocaine (Acute Coc). The shell division sampled within the nucleus accumbens is indicated by the white ellipse. Scale bar indicates 100 microns. Panel c: the mean (+SEM) number of Fos positive nuclei in the nucleus accumbens (+2.0 mm from bregma) core and shell subdivisions two hours after an i.p. injection of cocaine (10 mg/kg) in rats with a history of cocaine self-administration, when cocaine was delivered i.v. over 5, 45 or 90 sec. Control rats with no history of cocaine self-administration were given an i.p. injection of either saline (S) or cocaine for the first time (C). This test was conducted 45 days after the last self-administration session. In the NAc shell, a cocaine priming injection produced greater Fos expression compared to the saline control group in all groups except the 5 sec group (45 sec, p<0.004; 90 sec, p<0.007; acute cocaine control group, p<0.008). The 45, 90 sec and acute cocaine groups did not differ from each other, and these groups all showed significantly greater Fos expression than the 5 sec group (5 sec vs. 45 sec, p<0.028; vs. 90 sec, p<0.047; vs. acute cocaine control, p=0.059). In the NAc core, only the 45 sec (p<0.001), 90 sec (p<0.02) and acute cocaine group (p<0.02) had greater Fos expression compared to the saline control group, The 5 sec group did not have greater Fos expression than saline control (p>0.05), and had significantly fewer Fos positive cells than all other groups given cocaine priming injections (p’s<0.02).
In the NAc shell, a one-way ANOVA on the number of Fos positive nuclei revealed a main effect of group (F(4,34)=3.901, p<0.011). Relative to rats given saline, cocaine increased the number of Fos positive cells in all groups, except in the 5 sec group. The 45 sec, 90 sec and acute cocaine control groups did not differ from one another, and all showed significantly greater Fos expression than the 5 sec group. For the NAc core, a one-way ANOVA also indicated there were significant group differences in the number of Fos positive cells (F(4,34)=8.168 p<0.001). Compared to the saline control group, a cocaine injection increased the number of Fos positive cells in the 45 sec, 90 sec and acute cocaine control group, and these groups did not differ from one another. In contrast, cocaine failed to increase Fos expression in the 5 sec group, and the 5 sec group had significantly fewer Fos positive cells than all the other groups given cocaine.
In summary, the ability of a cocaine challenge injection to induce Fos 45 days after the last self-administration session was markedly suppressed in rats with a history of self administering cocaine injected over 5 sec, but not in rats with a history of self administering cocaine injected over either 45 or 90 sec. There is, therefore, at least one long term change in the nucleus accumbens produced by a previous history with self administered cocaine when it is administered rapidly (over 5 sec) that is not evident when it is administered more slowly (45 – 90 sec).
Discussion
Few people who try drugs become addicted (Wagner and Anthony, 2002), and many factors influence addiction liability. Here we focused on one – how fast drug reaches the brain. The faster drugs reach the brain the greater the liability to addiction (Jones, 1990; Gorelick, 1992; Hatsukami and Fischman, 1996; Karch, 1999), although why this is so is not known. To determine if variation in the rate of cocaine delivery influences self-administration behavior we varied the rate it was injected between 5 and 90 sec. This range captures rates that addicts report when injecting drugs (Zernig et al., 2003), approximates the difference in pharmacokinetics between snorting and smoking (Jones, 1990), and influences the subjective effects of cocaine (Abreu et al., 2001) – while not influencing peak brain levels of cocaine (Samaha et al., 2002; Shou et al., 2006). We assessed self-administration first under limited access conditions (1 hr/day), and then when rats were given 6-fold more time to take cocaine (6 hrs/day). We then tested the ability of an i.p. priming injection of cocaine to reinstate drug-seeking behavior relatively soon after extended access self-administration, and again after a long period of abstinence.
Rapid cocaine delivery promotes a large increase in drug intake only when rats are given extended access
Varying the rate in cocaine delivery could influence self-administration behavior because this introduces a delay between an action and the receipt of cocaine, which could theoretically impact its reinforcing effects. However, this does not seem to be the case under the conditions in this study, because there was only a modest and statistically insignificant effect of varying the rate of i.v. cocaine delivery between 5 and 90 sec on drug intake under limited access conditions (1 hr/day). This is consistent with previous studies in rats; variation in the rate of cocaine delivery over this range has no effects on the acquisition or maintenance of cocaine self-administration behavior, on progressive ratio performance or on reinstatement (Pickens et al., 1969; Liu et al., 2005; Crombag et al., 2008). Furthermore, it is consistent with studies in rhesus monkeys in which varying the rate of cocaine delivery between 10 and 600 sec had no effect on the ED50 for maintaining cocaine self-administration on a progressive ratio schedule (Woolverton and Wang, 2004). Similarly, rats self-administer nicotine delivered over a wide range of speeds, and in fact, prefer slower speeds (Sorge and Clarke, 2009). Thus, as concluded by Liu et al. (2005), “injection duration did not have profound effects on the acute reinforcing effects of cocaine” (p. 195; also see, Pickens et al., 1969; Crombag et al., 2008).
However, when given more opportunity to take cocaine there was a striking effect on the amount of drug consumed. When given 6-fold more time to take drug rats receiving cocaine rapidly (5 – 45 sec) increased their intake 8-fold. In contrast, when cocaine was delivered over 90 sec rats increased their intake only 2-fold. This suggests that the rate that drug reaches the brain is a major factor influencing escalation of use – an important symptom of addiction. Given that this escalation in intake is not because rapid injections are simply more reinforcing, there must be another mechanism.
Rats that in the past experienced rapidly delivered cocaine show greater reinstatement after a long period of abstinence
In addition to escalation of intake, another symptom of addiction is a high propensity to relapse. We found that after a short period of abstinence (15 days) an i.p drug “prime” reinstated responding in all groups, but after a longer period of abstinence (45 days) only rats that previously had cocaine injected over 5 sec reinstated responding.
It is not clear why after prolonged abstinence only rats that previously experienced cocaine delivered over 5 sec reinstated drug-seeking, but it is possible that rapid drug delivery produces more persistent changes in brain systems that mediate the incentive motivational effects of drugs. Consistent with this hypothesis, we found group differences in the ability of the i.p. cocaine prime to induce the expression of the immediate early gene, c-fos, in the nucleus accumbens. Repeated exposure to cocaine produces a marked desensitization in its ability to induce Fos protein (e.g. Moratalla et al., 1996; Ben-Shahar et al., 2004; Zahm et al., 2009), and here this effect persisted only in rats with a history of 5 sec infusions. In rats that previously received cocaine over 45 or 90 sec the cocaine prime induced similar Fos expression in the nucleus accumbens as in drug naïve controls. We have seen a similar effect of rate of infusion after the repeated administration of nicotine (Samaha et al., 2005). This result suggests that rapidly administered cocaine has more persistent effects on both brain and on behavior than when cocaine reaches the brain even a little slower. Furthermore, the persistent effect of rapidly administered cocaine is not just a consequence of the amount of drug previously taken, as rats in the 5 and 45 sec groups did not differ in total drug intake.
How persistent desensitization of cocaine-induced Fos expression might contribute to an increased propensity to reinstatement is unknown, and there may be no causal relationship. Chronic cocaine does result in an accumulation of the transcription factor ΔFosB (Hope et al. 1994; Kelz et al., 1999; Colby et al., 2003; McClung et al., 2004), and ΔFosB can suppress the transcription of c-fos mRNA through chromatin remodeling (Renthal et al., 2008). Perhaps the persistent desensitization of c-fos after 5 sec injections reflects a continued elevation in ΔFosB, which is thought to be important in addiction (Nestler, 2001). However, this is highly speculative and the mechanism responsible for the influence of speed of cocaine delivery on the escalation in intake and persistent susceptibility to reinstatement described here remains to be determined.
Although these experiments do not establish why the rate of cocaine delivery had such a profound effect on the amount of drug intake and susceptibility to reinstatement, we can exclude some obvious potential causes. First, the long “time out” interval did not constrain self-administration behavior (Fig. 2), and all rats had exactly the same opportunity to take drug. It is not surprising that the 90 sec interval required between injections had no effect on self-administration behavior because this is far shorter than the “self-imposed” inter-infusion interval seen under these conditions, which is approximately 4 min (also see Mantsch et al., 2001; Ferrario et al., 2005, Crombag et al., 2008; Belin et al., 2009). Another possibility is that the amount of drug to reach the brain varies across these infusion rates, but this is not the case. Varying the rate of i.v. cocaine delivery between 5 and 100 sec has no effect on peak brain levels of cocaine (see Fig. 1 in Samaha et al., 2002; also Shou et al. 2006 and Schindler et al., 2009) – all that differs is the time to reach peak. This is consistent with the observation that the peak amount of dopamine overflow in the striatum measured with rapid microdialysis sampling is the same in rats administered i.v. cocaine over 5 or over 100 sec (Ferrario et al., 2008).
Implications for addiction
Whatever the underlying mechanism, our results establish that when provided with the opportunity to take large amounts of drug, the rapid delivery of cocaine preferentially produces a marked escalation in intake. This could obviously account for why cocaine is more addictive when it reaches the brain rapidly, for example, when it is smoked rather than snorted (Jones, 1990; Hatsukami and Fischman, 1996). An escalation of intake would result in the brain being exposed to much larger amounts of drug, which presumably would further facilitate many different changes in the body and brain that could contribute to addiction. There are many classes of adaptations that could be involved – from homeostatic adaptations related to tolerance to those related to incentive sensitization. Consistent with the latter, the rapid administration of cocaine and nicotine facilitates the development of psychomotor sensitization, and therefore the associated changes in brain responsible for this form of experience-dependent plasticity (Samaha et al., 2002; Samaha et al., 2005). Furthermore, when administered acutely, the rapid administration of cocaine (Samaha et al., 2004) and nicotine (Samaha et al., 2005) is more effective in inducing c-fos and arc mRNA in mesocorticolimbic structures, and even engages different cells and circuits than when administered even a little slower (also see Kiyatkin and Brown, 2005). Therefore, it is possible that rapid injections promote adaptations in brain regions that mediate incentive motivation for drug, increasing degree to which the drug is “wanted”, further increasing intake.
In conclusion, we suggest that cocaine is potentially more addictive when taken in forms or routes that result in its rapid entry into the brain because: (1) this promotes a marked escalation in drug intake, and (2) it renders individuals more susceptible to relapse, even long after the discontinuation of drug use. These effects are presumably because the rapid delivery of cocaine promotes persistent changes in brain systems that regulate motivation for drug, and the continuing exposure to high levels of drug may result in a vicious cycle that further promotes maladaptive changes in brain and behavior.
Acknowledgements
This research was supported by National Institute on Drug Abuse (NIDA) Grant R37 DA004294 (T.E.R.). K.T.W. was supported by an NIDA Individual National Research Service Award (NRSA) F31 DA021060. We would like to thank Drs. Hans Crombag, Sheila M. Reynolds, and Anna-Noel Samaha for helpful discussions.
References
- Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology (Berl) 2001;154:76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration. J Exp Anal Behav. 1973;20:119–129. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Balado E, Piazza PV, Deroche-Gamonet V. Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol Psychiatry. 2009;65:863–868. doi: 10.1016/j.biopsych.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Posthumus EJ, Waldroup SA, Ettenberg A. Heightened drug-seeking motivation following extended daily access to self-administered cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:863–869. doi: 10.1016/j.pnpbp.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Kiyatkin EA. Brain temperature change and movement activation induced by intravenous cocaine delivered at various injection speeds in rats. Psychopharmacology (Berl) 2005;181:299–308. doi: 10.1007/s00213-005-2244-0. [DOI] [PubMed] [Google Scholar]
- Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine. J Neurosci. 2003;23:2488–2493. doi: 10.1523/JNEUROSCI.23-06-02488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Robinson TE. Signalled versus unsignalled intravenous amphetamine: large differences in the acute psychomotor response and sensitization. Brain Res. 1996;722:227–231. doi: 10.1016/0006-8993(96)00066-2. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Ferrario CR, Robinson TE. The rate of intravenous cocaine or amphetamine delivery does not influence drug-taking and drug-seeking behavior in rats. Pharmacol Biochem Behav. 2008;90:797–804. doi: 10.1016/j.pbb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunais JB, McGinty JF. Acute and chronic cocaine administration differentially alters striatal opioid and nuclear transcription factor mRNAs. Synapse. 1994;18:35–45. doi: 10.1002/syn.890180106. [DOI] [PubMed] [Google Scholar]
- Daunais JB, Roberts DC, McGinty JF. Short-term cocaine self administration alters striatal gene expression. Brain Res Bull. 1995;37:523–527. doi: 10.1016/0361-9230(95)00049-k. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci. 1998;10:3565–3571. doi: 10.1046/j.1460-9568.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for Addiction-like Behavior in the Rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Shou M, Samaha AN, Watson CJ, Kennedy RT, Robinson TE. The rate of intravenous cocaine administration alters c-fos mRNA expression and the temporal dynamics of dopamine, but not glutamate, overflow in the striatum. Brain Res. 2008;1209:151–156. doi: 10.1016/j.brainres.2008.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA. Progression of dependence in male cocaine addicts. Am J Drug Alcohol Abuse. 1992;18:13–19. doi: 10.3109/00952999209001607. [DOI] [PubMed] [Google Scholar]
- Gorelick DA. The rate hypothesis and agonist substitution approaches to cocaine abuse treatment. Adv Pharmacol. 1998;42:995–997. doi: 10.1016/s1054-3589(08)60914-x. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Fischman MW. Crack cocaine and cocaine hydrochloride.Are the differences myth or reality? Jama. 1996;276:1580–1588. [PubMed] [Google Scholar]
- Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- Hope BT, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci U S A. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Jones RT. The pharmacology of cocaine smoking in humans. NIDA Res Monogr. 1990;99:30–41. [PubMed] [Google Scholar]
- Karch SB. Cocaine: history, use, abuse. J R Soc Med. 1999;92:393–397. doi: 10.1177/014107689909200803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Wakasa Y, Yanagita T. Relationship between minimum reinforcing doses and injection speed in cocaine and pentobarbital self-administration in crab-eating monkeys. Pharmacol Biochem Behav. 1987;28:407–410. [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Dopamine-dependent and dopamine-independent actions of cocaine as revealed by brain thermorecording in freely moving rats. Eur J Neurosci. 2005;22:930–938. doi: 10.1111/j.1460-9568.2005.04269.x. [DOI] [PubMed] [Google Scholar]
- Kowalchuk RK, Keselman HJ. Mixed-model pairwise multiple comparisons of repeated measures means. Psychol Methods. 2001;6:282–296. doi: 10.1037/1082-989x.6.3.282. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roberts DC, Morgan D. Sensitization of the reinforcing effects of self-administered cocaine in rats: effects of dose and intravenous injection speed. Eur J Neurosci. 2005;22:195–200. doi: 10.1111/j.1460-9568.2005.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Ho A, Schlussman SD, Kreek MJ. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl) 2001;157:31–39. doi: 10.1007/s002130100744. [DOI] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Elibol B, Vallejo M, Graybiel AM. Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron. 1996;17:147–156. doi: 10.1016/s0896-6273(00)80288-3. [DOI] [PubMed] [Google Scholar]
- Nelson RA, Boyd SJ, Ziegelstein RC, Herning R, Cadet JL, Henningfield JE, Schuster CR, Contoreggi C, Gorelick DA. Effect of rate of administration on subjective and physiological effects of intravenous cocaine in humans. Drug Alcohol Depend. 2006;82:19–24. doi: 10.1016/j.drugalcdep.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- O'Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology. 2005;30:1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR, Gilman JP, Jufer R, Cone EJ, Schindler CW. Effects of delivery rate and non-contingent infusion of cocaine on cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl) 1998;137:253–258. doi: 10.1007/s002130050618. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. Edition. New York: Academic Press; 1998. [Google Scholar]
- Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology (Berl) 2007;194:127–137. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- Pickens R, Dougherty J, Thompson T. Effects of volume and duration of infusion on cocaine reinforcement with concurrent activity recording; Minutes of the Meeting of the Committee on Problems of Drug Dependence; Washington, D.C.: NAS-NRC; 1969. pp. 5805–5811. [Google Scholar]
- Renthal W, Carle TL, Maze I, Covington HE, 3rd, Truong HT, Alibhai I, Kumar A, Montgomery RL, Olson EN, Nestler EJ. Delta FosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J Neurosci. 2008;28:7344–7349. doi: 10.1523/JNEUROSCI.1043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick RB, Kestenbaum RS, Schwartz LK. Acute systemic effects of cocaine in man: a controlled study by intranasal and intravenous routes. Science. 1977;195:696–698. doi: 10.1126/science.841307. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci. 2008;11:423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Robinson TE. Why does the rapid delivery of drugs to the brain promote addiction? Trends Pharmacol Sci. 2005;26:82–87. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Li Y, Robinson TE. The rate of intravenous cocaine administration determines susceptibility to sensitization. J Neurosci. 2002;22:3244–3250. doi: 10.1523/JNEUROSCI.22-08-03244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Yau WY, Yang P, Robinson TE. Rapid delivery of nicotine promotes behavioral sensitization and alters its neurobiological impact. Biol Psychiatry. 2005;57:351–360. doi: 10.1016/j.biopsych.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Mallet N, Ferguson SM, Gonon F, Robinson TE. The rate of cocaine administration alters gene regulation and behavioral plasticity: implications for addiction. J Neurosci. 2004;24:6362–6370. doi: 10.1523/JNEUROSCI.1205-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Thorndike EB. Effect of rate of delivery of intravenous cocaine on self-administration in rats. Pharmacol Biochem Behav. 2009;93:375–381. doi: 10.1016/j.pbb.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shou M, Ferrario CR, Schultz KN, Robinson TE, Kennedy RT. Monitoring dopamine in vivo by microdialysis sampling and on-line CE-laser-induced fluorescence. Anal Chem. 2006;78:6717–6725. doi: 10.1021/ac0608218. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Clarke PB. Rats self-administer intravenous nicotine delivered in a novel smoking-relevant procedure: effects of dopamine antagonists. J Pharmacol Exp Ther. 2009;330:633–640. doi: 10.1124/jpet.109.154641. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Weeks J. Long-term intravenous infusions. London: Academic Press; 1972. [Google Scholar]
- Weeks JR, Davis JD. Chronic Intravenous Cannulas For Rats. J Appl Physiol. 1964;19:540–541. doi: 10.1152/jappl.1964.19.3.540. [DOI] [PubMed] [Google Scholar]
- West BW, KB Galecki AT. Linear Mixed Models: A Practical Guide Using Statistical Software. 2nd Edition. Boca Raton: Chapman & Hill/CRC; 2007. [Google Scholar]
- Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength of cocaine. Eur J Pharmacol. 2004;486:251–257. doi: 10.1016/j.ejphar.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Becker ML, Freiman AJ, Strauch S, Degarmo B, Geisler S, Meredith GE, Marinelli M. Fos After Single and Repeated Self-Administration of Cocaine and Saline in the Rat: Emphasis on the Basal Forebrain and Recalibration of Expression. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Giacomuzzi S, Riemer Y, Wakonigg G, Sturm K, Saria A. Intravenous drug injection habits: drug users' self-reports versus researchers' perception. Pharmacology. 2003;68:49–56. doi: 10.1159/000068731. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang L, Tang Y, Zhang Q, Lou D, Sharp FR, Zhang J, Xu M. Repeated Cocaine Administration Induces Gene Expression Changes through the Dopamine D1 Receptors. Neuropsychopharmacology. 2005;30:1443. doi: 10.1038/sj.npp.1300680. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang L, Jiao H, Zhang Q, Zhang D, Lou D, Katz JL, Xu M. c-Fos facilitates the acquisition and extinction of cocaine-induced persistent changes. J Neurosci. 2006;26:13287–13296. doi: 10.1523/JNEUROSCI.3795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]