Figure 1. Expression of the SH3A of ITSN-1 in cultured ECs impairs caveolae internalization.
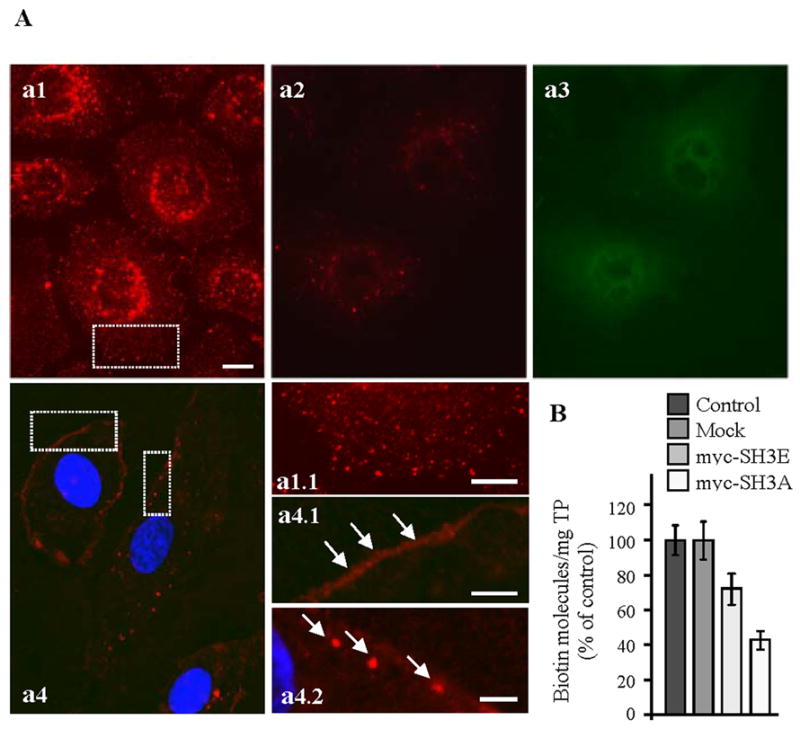
(A) In control cells subjected to biotinylation of cell surface proteins and internalization assay (37°C, 30 min), neutrAvidin-Texas Red staining indicates a strong punctate pattern throughout the cytosol (a1, a1.1), with accumulation in the perinuclear area. ECs transiently transfected with myc-SH3A, selected based on their resistance to blasticidin, and then subjected to the internalization assay using cleavable biotin show limited neutrAvidin-Texas Red staining and some accumulation in the perinuclear area (a2). Anti-myc mAb followed by anti-mouse IgG Alexa Fluor488-conjugated was used to visualize transfected cells (a3). Use of non-cleavable biotin demonstrates sequestration of biotinylated proteins at the cell surface (a4, a4.1 arrows) and limited internalization. Within cells, biotin was often detected in large puncta under the PM (a4.2, arrows). Bars: 10 μm a1–a4; 5 μm – a1.1, a4.1; 3 μm - a4.2. (B) The number of biotin molecules present in ECs lysates prepared from controls, mock myc-SH3A and myc-SH3E transfected ECs was determined by ELISA in 3 – 4 different experiments. Ordinate: number of biotin molecules normalized per mg total protein (TP). Bars ± SD.
