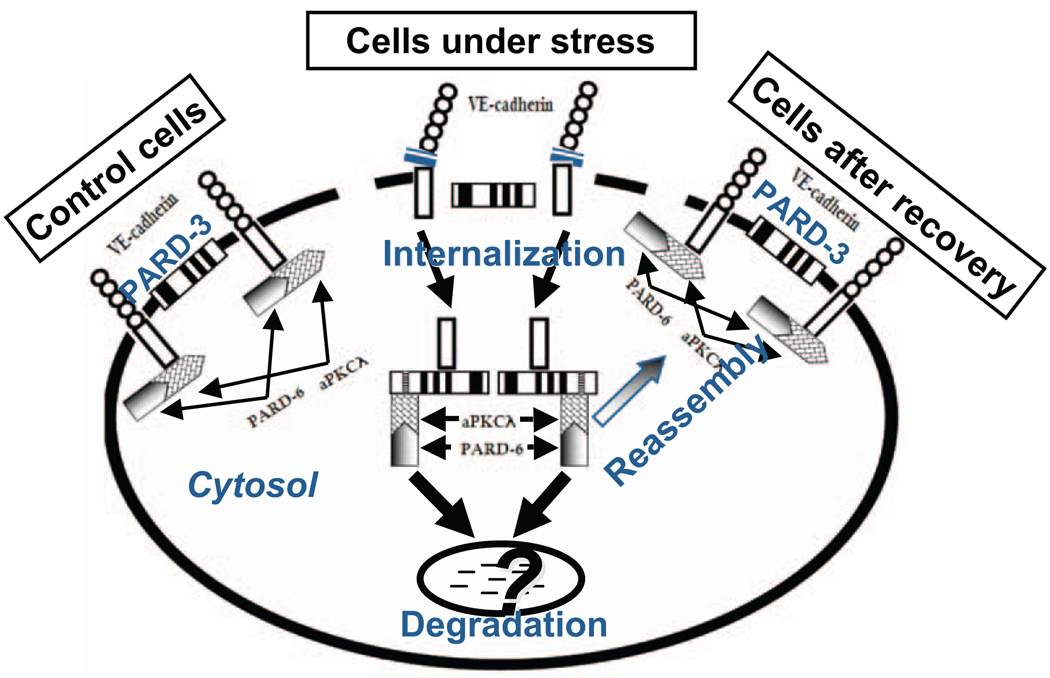
Figure 3.
Proposed hypothesis that illustrate interactions between VE-cadherin, partitioning defective-3 (PARD-3), and atypical protein kinase C (aPKC) in control cells and cells under stress. In quiescent control cells, endothelial junction structure is intact. VE-cadherin forms a complex with aPKCλ, which interacts with PARD-6. Although both VE-cadherin and PARD-3 are located at cell junction, the 2 molecules have no interaction. However, when cells are under stress conditions, such as when cells were treated with preeclamptic conditioned medium (PE-CM), expression and distribution of VE-cadherin and PARD-3 are reduced at cell junction, and internalized in cytosol. The event is accompanied with dissociation of VE-cadherin and aPKCλ interactions. Dissociated VE-cadherin and aPKCλ then interact with PARD-3 and form VE-cadherin/PARD-3 and aPKCλ/PARD-3 complexes. At present it is not known whether VE-cadherin/PARD-3 and aPKCλ/PARD-3 complex formations are associated with cell polarity changes or this process is a mechanism that protects VE-cadherin or PARD-3 from degradation by proteolysis. However, it is clear that the changes of the protein-protein interactions between VE-cadherin and PARD-3 and aPKCλ and PARD-3 are transient and only occur when cells are under stress challenge. Because after eliminating the stimuli (CM), interactions between VE-cadherin and PARD-3 and aPKCλ and PARD-3 no longer exist, that is, after recovery, expression and distribution of VE-cadherin and PARD-3 at intercellular junction and the interaction between VE-cadherin and aPKC and PARD-3 return to the control cell levels.