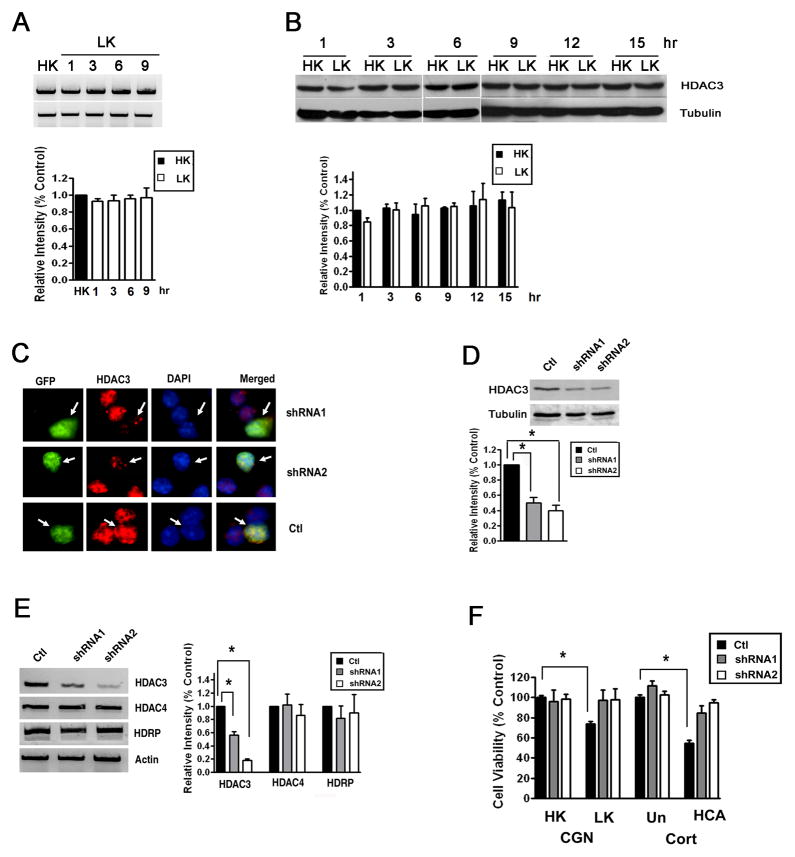
Figure 2. Suppression of HDAC3 expression protects neurons.
A) CGNs were treated with LK for 1, 3, 6 or 9 hr. Control cultures received HK for 9 h (HK). Total RNA was extracted and RT-PCR performed using specific primers for HDAC3. Actin served as loading control. The right panel shows the densitometric analysis of the RT-PCR data from three separate experiments.
B) CGNs were treated with HK or LK medium for 1, 3, 6, 9, 12 or 15 hr. Expression of HDAC3 protein was analyzed by Western blotting. The blot was reprobed with a tubulin antibody. Densitometric analysis of the bands from different experiments was performed and normalized to tubulin.
C) CGNs were cotransfected with plasmids expressing GFP with either control shRNA (Ctl), shRNA-1, and shRNA-2 for 72 hr. The cells were co-stained with GFP and HDAC3 antibodies and nuclear morphology visualized by DAPI staining. Arrows point to successfully-transfected cells (GFP-positive). Neurons receiving either shRNA construct show reduced HDAC3 immunostaining whereas cells cotransfected with the control shRNA show similar HDAC3 staining as untransfected cells.
D and E) Lysates from HT22 cells transfected with control shRNA (Ctl), shRNA-1, and shRNA-2 were analyzed by Western blotting or RT-PCR. Panel D shows Western blot results using HDAC3 and tubulin antibodies. Densitometric analysis of the bands was performed and normalized to tubulin. Panel C shows results of RT-PCR analysis of HDAC3, HDAC4, HDRP and actin (loading control) expression. Also included is densitometric analysis of the data from three different experiments normalized to actin.
F) CGNs and cortical neurons (Cort) were transfected with plasmids expressing control shRNA (Ctl), shRNA-1, and shRNA-2 and cell viability quantified as described in Methods. After transfection, CGNs were treated with HK or LK for 24 hr whereas cortical neurons (Cort) were either untreated (Un) or treated with HCA for 20 hr. Cell viability is normalized to survival in cultures transfected with control shRNA and treated in HK (CGN) or untreated (Cort). * p < 0.05