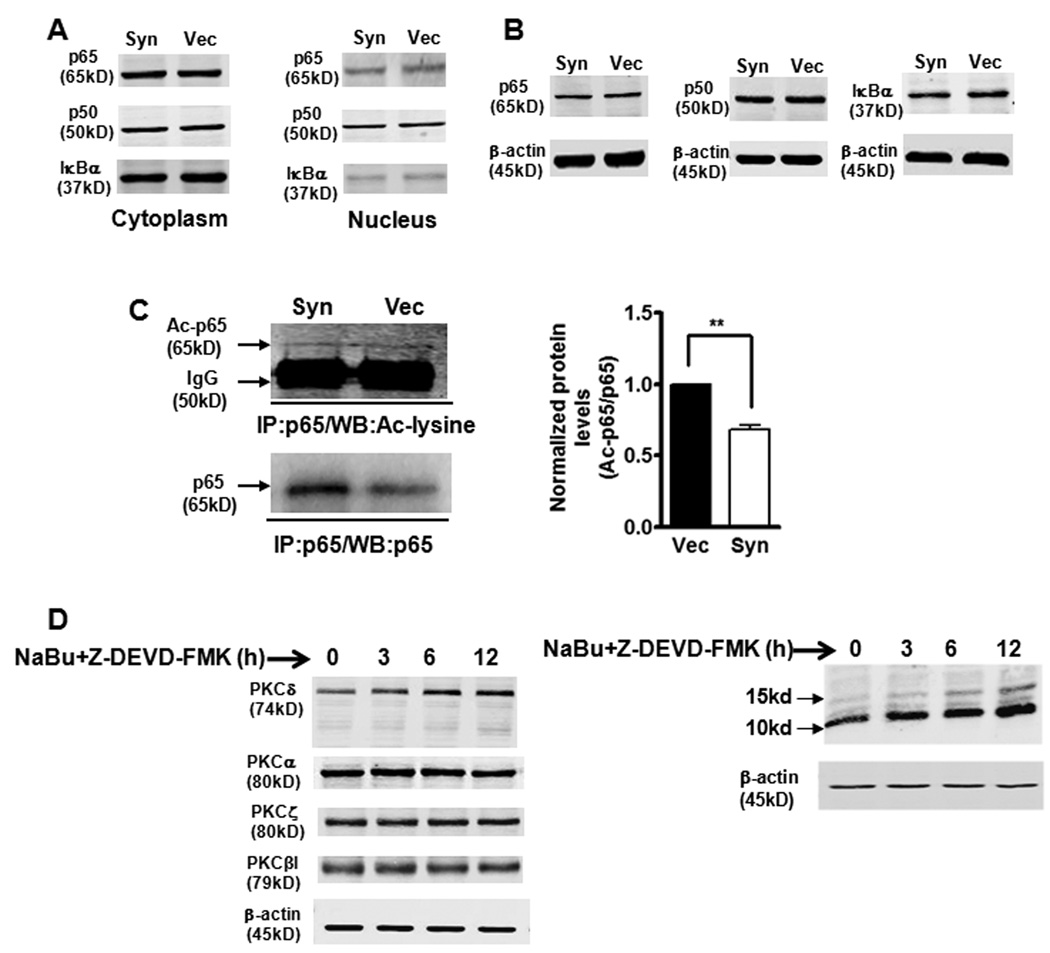
Figure 7.
α-Synuclein-induced blockade of NFκB activation is associated with decreased acetylation of p65, but does not correlate with nuclear translocation or protein levels of NFκB/IκBα. A, B, Nuclear translocation and abundance of NFκB/IκBα were not altered by overexpression of αsyn. Representative immunoblot of p65, p50 and IκBα levels on cytoplasmic and nuclear extracts (A) or whole cell lysates (B) from αsyn (Syn) and vector control (Vec) cells. C, The p65 acetylation levels were reduced in αsyn cells. Whole cell lysates was immunoprecipitated (IP) with p65 antibody. The resulting immunoprecipitates were blotted with anti-acetyl-lysine and anti-p65 antibodies. Densitometric quantitation of the ratio of band intensity of acetylated p65 and total p65 from two independent experiments (means ± SEM; **p<0.01) is shown on the right. D, Sodium butyrate (NaBu) specifically enhanced PKCδ isoform expression in αsyn-expressing N27 cells. αSyn-expressing cells were treated with 1 mM NaBu and 50 µM caspase-3 inhibitor Z-DEVD-FMK, and cell lysates were prepared for blotting with specific anti-PKC isoforms (left panel) and anti-acetyl-lysine (right panel) antibodies.