Abstract
The purpose of this study was to examine the effects of forced mouth opening on murine mandibular condylar head remodeling. We hypothesized that forced mouth opening would cause an anabolic response in the mandibular condylar cartilage. Six-week-old female C57BL/6 mice were divided into 3 groups: (1) control, (2) 0.25 N, and (3) 0.50 N of forced mouth opening. Gene expression, micro-CT, and proliferation were analyzed. 0.5 N of forced mouth opening caused a significant increase in mRNA expression of Pthrp, Sox9, and Collagen2a1, a significant increase in proliferation, and a significant increase in trabecular spacing in the subchondral bone, whereas 0.25 N of forced mouth opening did not cause any significant changes in any of the parameters examined. Forced mouth opening causes an increase in the expression of chondrocyte maturation markers and an increase in subchondral trabecular spacing.
Keywords: temporomandibular joint, subchondral bone, mouse models, chondrocyte maturation
INTRODUCTION
The articulating surfaces of the temporomandibular joint (TMJ) are covered by fibrocartilage (Benjamin and Ralphs, 2004), which is composed of chondrocytes at various stages of maturation that are organized into four zones: (1) superior articular zone—cells in this zone express proteoglycan 4 (Prg4) (Ohno et al., 2006); (2) polymorphic zone—cells in this zone express SRY-box 9 (Sox9) and parathyroid-hormone-related protein (Pthrp); (3) flattened zone—the cartilage cells in this layer express collagen type II (Col 2a1) (Shibukawa et al., 2007); and (4) hypertrophic zone (Shibukawa et al., 2007)—the chondrocytes express collagen type X (Col 10a1) (Shibukawa et al., 2007). Growth in the condylar cartilage proceeds from cells exiting the proliferative pool in the polymorphic zone and undergoing endochondral ossification in the inferior cartilage zones (Luder et al., 1988). The mandibular condylar cartilage is able to remodel its structure in response to mechanical strains (Luder et al., 1988; Wadhwa and Kapila, 2008). The remodeling is achieved by the generation of new chondrocytes and alterations in the composition of the extracellular matrix to achieve a better balance between mechanical stress and the load-bearing capacity of the joint (Shen and Darendeliler, 2005). Mechanical loading-induced TMJ remodeling is important, because it is the basis of functional appliance therapies in orthodontics (Rabie and Hägg, 2002; Tang et al., 2004), and defects in the process have been linked to degenerative diseases of the TMJ (Milam, 2005; Tanaka et al., 2008).
Because of transgenic mouse technology, a murine model of mechanical loading-induced TMJ remodeling is advantageous, because it will facilitate the elucidation of the roles of specific genes in the TMJ mechanotransduction pathway. In rabbits and rats, forced mouth opening TMJ remodeling has been described (Fujisawa et al., 2003; Tanaka et al., 2005; Kawai et al., 2008). The advantages of the forced mouth opening model compared with other mechanical loading TMJ remodeling models are that it is noninvasive, and the amount of external loading is finite and quantifiable. Since there are no reports of forced mouth opening in mice, the goal of this study was to develop a murine forced mouth opening TMJ remodeling model.
We recently described a murine decreased occlusal loading–induced TMJ remodeling model. In this model, we found that soft diet in combination with incisor trimming caused a reduction in proliferation and in the expression of mandibular condylar cartilage maturation markers (Chen et al., 2009b). Therefore, we hypothesized that increased loading by forced mouth opening will cause the opposite effects in the mandibular condylar cartilage, namely, an increase in proliferation and expression of chondrocyte maturation markers. The use of this model with transgenic mice may shed insight into why certain individuals are predisposed to diseases of the TMJ and/or predisposed to being a positive or negative responder to mandibular growth modification appliances.
MATERIALS & METHODS
Mice
All experiments were performed under an institutionally approved protocol for the use of animals in research (University of Connecticut Health Center #2009-550). Six-week-old C57BL/6 wild-type (n = 48) female mice (Charles River Laboratories, Inc., Wilmington, MA, USA) were divided into 3 groups: (1) nonloaded for 5 days (n = 16), (2) 0.25 N of continuous forced mouth opening for 1 hr a day for 5 days (n = 16), and (3) 0.50 N of continuous forced mouth opening for 1 hr a day for 5 days (n = 16). Immediately after force application on the 5th day, the mice were sacrificed. For 6-week-old WT C57BL/6 female mice, the measured passive maximal mouth opening is 10 mm. Therefore, springs were custom-made with stainless steel wires 60 mm in length and 0.18–0.32 inch in diameter to deliver 0.25–0.5 N of force at 10 mm and 0 N at 14 mm of opening (Fig. 1A). Individual spring forces were measured with a mechanical test system (Electroforce 3200, Bose EnduraTec, Eden Prairie, MN, USA). Both control and forced mouth opening groups were anesthetized with ketamine (87 mg/kg)/xylazine (13 mg/kg) every day for the duration of the experiment. Three hrs prior to euthanasia, the mice were injected intraperitoneally with 0.1 mg bromodeoxyuridine (BrdU) per gram body weight. The mice were weighed and springs were recalibrated every day.
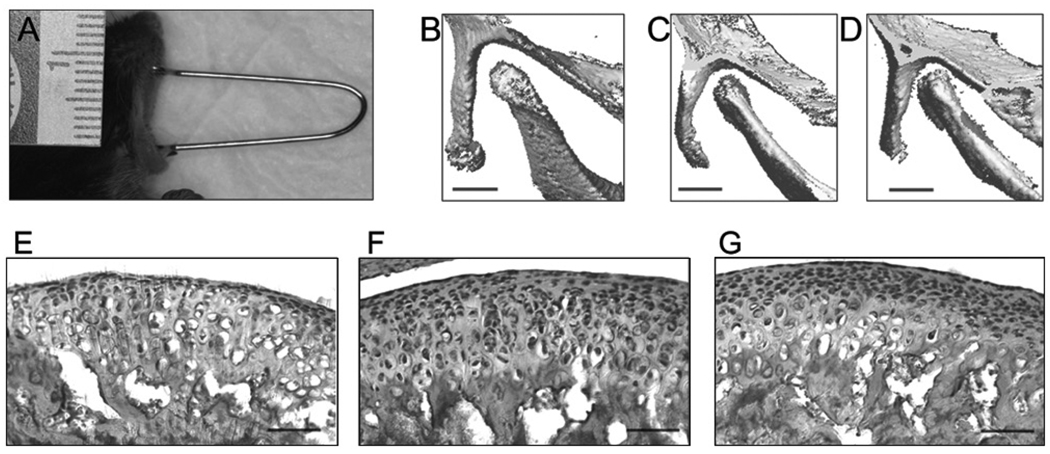
Figure 1.
Forced mouth opening model. (A) Image of anesthetized mouse exposed to forced mouth opening. (B,C,D) The region of skull including the mandibular condyle was imaged by micro-CT (VivaCT40, Scanco Medical AG, Bassersdorf, Switzerland), with the mouth in the closed position (B) and then opened with spring forces of 0.25 N (C) and 0.5 N (D). Bar: 500 µm. (E,F,G) H&E staining of sagittal sections of the TMJ in 47-day-old female C57BL/6 mice exposed to 5 days of 0 (E), 0.25 N (F), or 0.5 N (G) forced mouth opening for 1 hr/day. Bar: 50 µm.
RNA Extraction and PCR Amplification
For each mouse, the mandibular condylar heads (left and right) containing both the mandibular condylar cartilage and subchondral bone were carefully isolated with all the soft tissues removed under a dissecting microscope. Total RNA was obtained from the condylar heads from 8 mice per group and extracted with TRIzol Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) following the manufacturer’s protocol. Total RNA was converted and amplified to cDNA by an ABI High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s protocol. Primers for Real-time PCR were purchased from Applied Biosystems. Real-time PCR was performed for parathyroid-hormone-related protein (Pthrp—Mm00436057_m1), SRY-box containing gene 9 (Sox 9—Mm 00448840_m1), collagen type II (Col 2a1—Mm00491889_m1), collagen type X (Col 10a1—Mm00487041_m1), osteoprotegrin (Opg—Mm00435452_m1), Receptor Activator for Nuclear Factor κ B Ligand (RANKL—Mm00441908_m1), and dentin matrix acidic phosphoprotein 1 (Dmp 1—Mm00803833_g1), as we have previously described (Chen et al., 2009a,b).
Histology and Immunohistochemistry
TMJs were fixed for 5 days in 10% formalin at 4°C from 8 mice per group (left TMJ). Following fixation, TMJs were washed 3 times in 1X phosphate-buffered solution, placed in 30% sucrose for an additional 24 hrs, embedded in tissue-embedding medium (Cryomatrix, Thermo Shandon, Pittsburgh, PA, USA) on dry ice, and immersed in methyl butane for 10 to 15 min. Thirty 5-µm sagittal sections from lateral to medial portions of the TMJ structures were collected from each sample. The sections from the mid-third of the TMJ (approximately 10 sections) were processed for immunohistochemistry, and/or H&E staining.
BrdU immunohistochemical analysis was completed with a BrdU staining kit following the manufacturer’s instructions (Zymed Laboratories-Invitrogen Corporation, Carlsbad, CA, USA). Negative controls consisted of omitting the primary antibody step and incubating them with blocking solution. Images of proliferating cells in the mandibular condylar cartilage were taken with an Axiocam MRc digital camera. To quantify BrdU staining, we superimposed a rectangular box of fixed area on 20X images of each section, and calculated a labeling index (number of BrdU-positive cells/total number of cells). From 3 to 5 sections, corresponding to the same anatomical area (midsagittal), were counted for each animal, and the average of these sections was the BrdU labeling index given to that mouse.
Micro-CT Analysis
Bone morphometry of the mandibular condyle from 8 mice per group was quantified by cone-beam micro-focus x-ray computed tomography (μCT40, Scanco Medical AG, Bassersdorf, Switzerland). Serial tomographic images were acquired at 55 kV and 145 μA, with 2000 projections per rotation collected at 300 msec integration time. Three-dimensional 16-bit grayscale images were reconstructed with standard convolution back-projection algorithms with Shepp and Logan filtering. Segmentation of bone from marrow and soft tissue was performed in conjunction with a constrained Gaussian filter to reduce noise, applying a hydroxyapatite-equivalent density threshold of 480 mg/cm3. Volumetric regions were selected to include the mandibular condyle (see Fig. 4A). The bone volume fraction and thickness and spacing of the trabecular bone were measured as direct indications of changes with forced mouth opening.
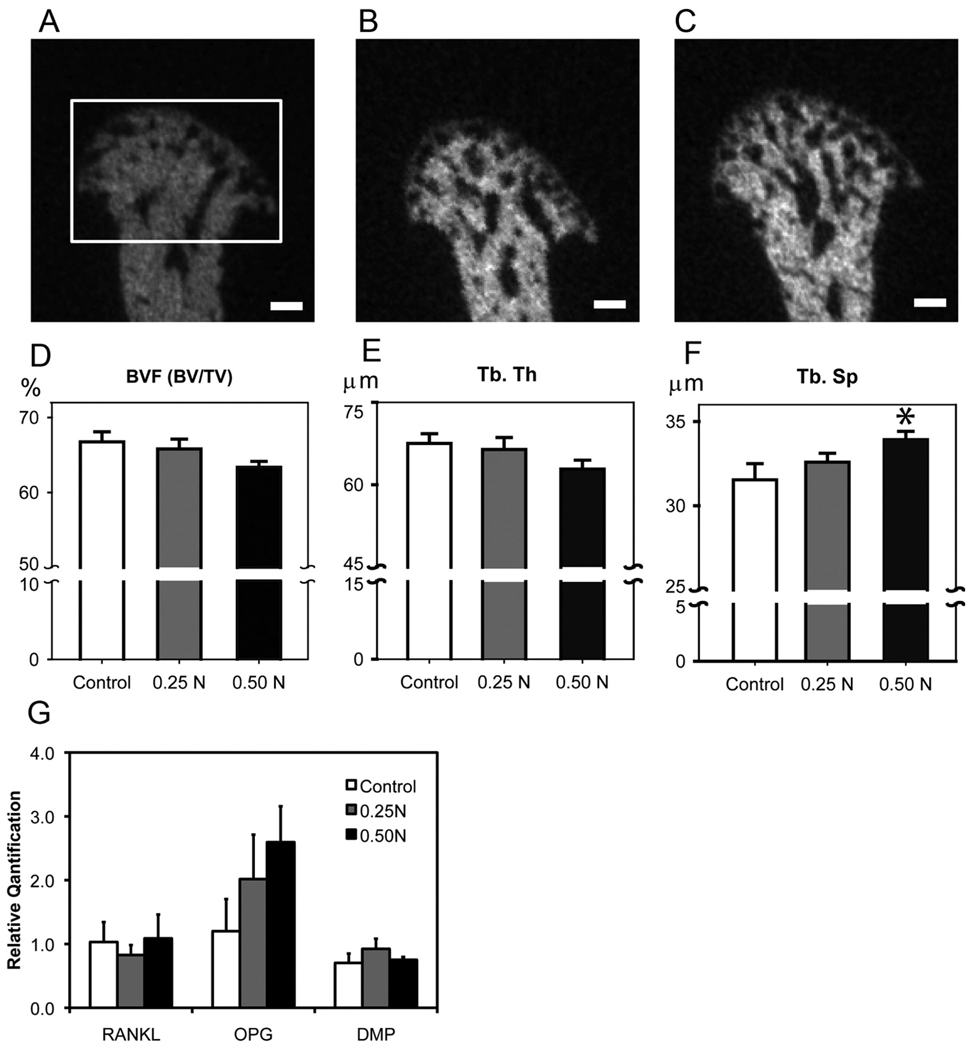
Figure 4.
Forced mouth opening causes an increase in trabecular spacing. (A,B,C) Micro-CT analysis of the subchondral bone. Representative images of mid-sagittal cross-sections from the mandibular condylar heads of 6-week-old female C57BL/6 mice exposed to (A) 0, (B) 0.25 N, and (C) 0.5 N of forced mouth opening. Bar: 100 µm. The framed area in (A) indicates the region of interest for the micro-CT analysis. (D,E,F) Micro-CT analysis of bone volume fraction (D), trabecular thickness (E), and trabecular spacing (F) from the mandibular condylar subchondral bone. (G) Real-time PCR analysis for RANKL, Opg, and Dmp-1 gene expressions from the mandibular condylar head. Points are the mean ± SE for n = 8 for each group. *Significant difference between 0.50 N forced mouth opening and control groups (p < 0.05).
Statistical Analysis
Statistical significance of differences among means was determined by analysis of variance (ANOVA), with post hoc comparison of more than two means by the Dunnett method using GraphPad Prism (San Diego, CA, USA).
RESULTS
There was no significant difference in the weight of the mice for the duration of the experiment for any of the experimental groups compared with the control group (data not shown). Micro-CT reconstructed three-dimensional images of the TMJ revealed a progressive decrease in the joint space with increasing magnitude of forced mouth opening (Figs. 1B, 1C, 1D). H&E-stained sections of the TMJ showed an apparent increase in cellularity in the superficial zones of the mandibular condylar cartilage in mice exposed to 0.5 N of forced mouth opening (Fig. 1G) compared with nonloaded controls (Fig. 1E).
Forced Mouth Opening Causes an Increase in Mandibular Condylar Cartilage Growth
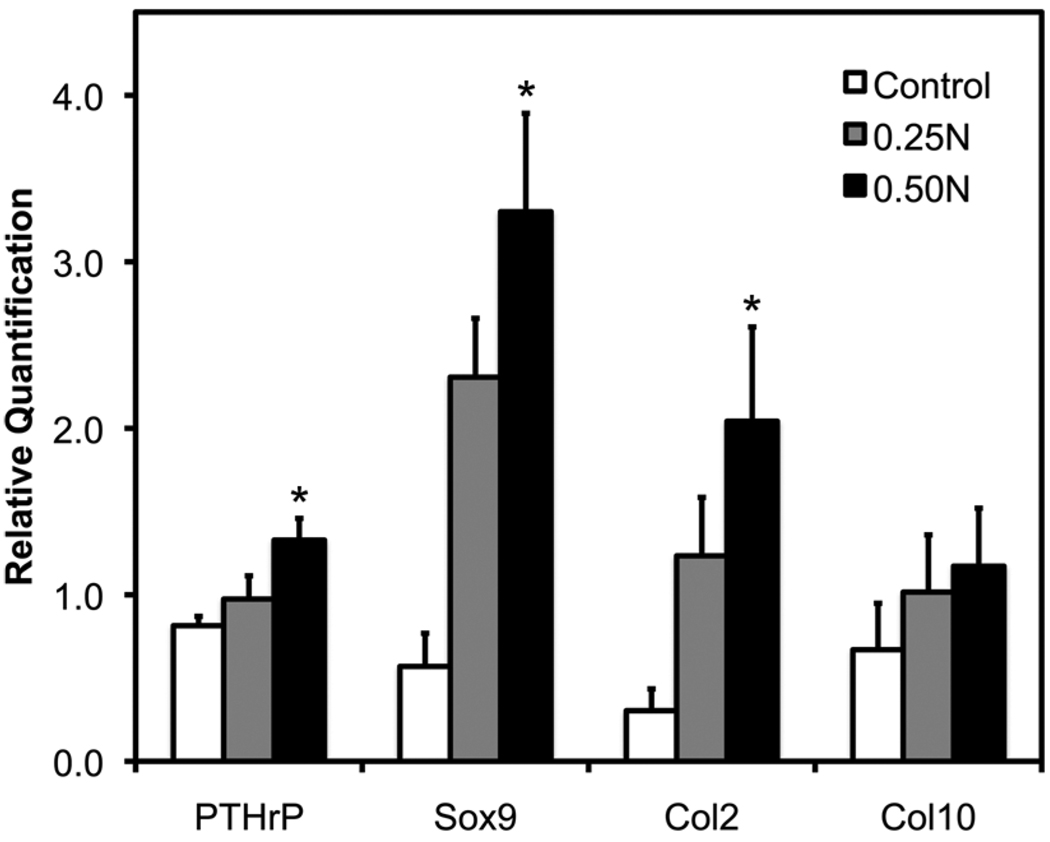
Cartilage phenotypic gene expression by qRT-PCR was performed with RNA isolated from the mandibular condylar head. 0.5 N of forced mouth opening for 1 hr a day for 5 days caused a significant increase in the mRNA expression of Pthrp, Sox9, and Col2a1 (Fig. 2) compared with nonloaded controls. Further, 0.25 N of forced mouth opening for 1 hr a day for 5 days caused a trend for increased Sox9 expression (p = 0.12) compared with nonloaded controls (Fig. 2).
Figure 2.
Gene expression of chondrocyte maturation markers in female C57BL/6 mice exposed to 0–0.5 N of forced mouth opening. Real-time PCR analysis was performed for gene expressions of Pthrp, Sox9, Col2a1, and Col10a1 from the mandibular condylar head. Points are the mean ± SE for n = 8 for each group. *Significant difference between forced mouth opening and control groups (P < 0.05).
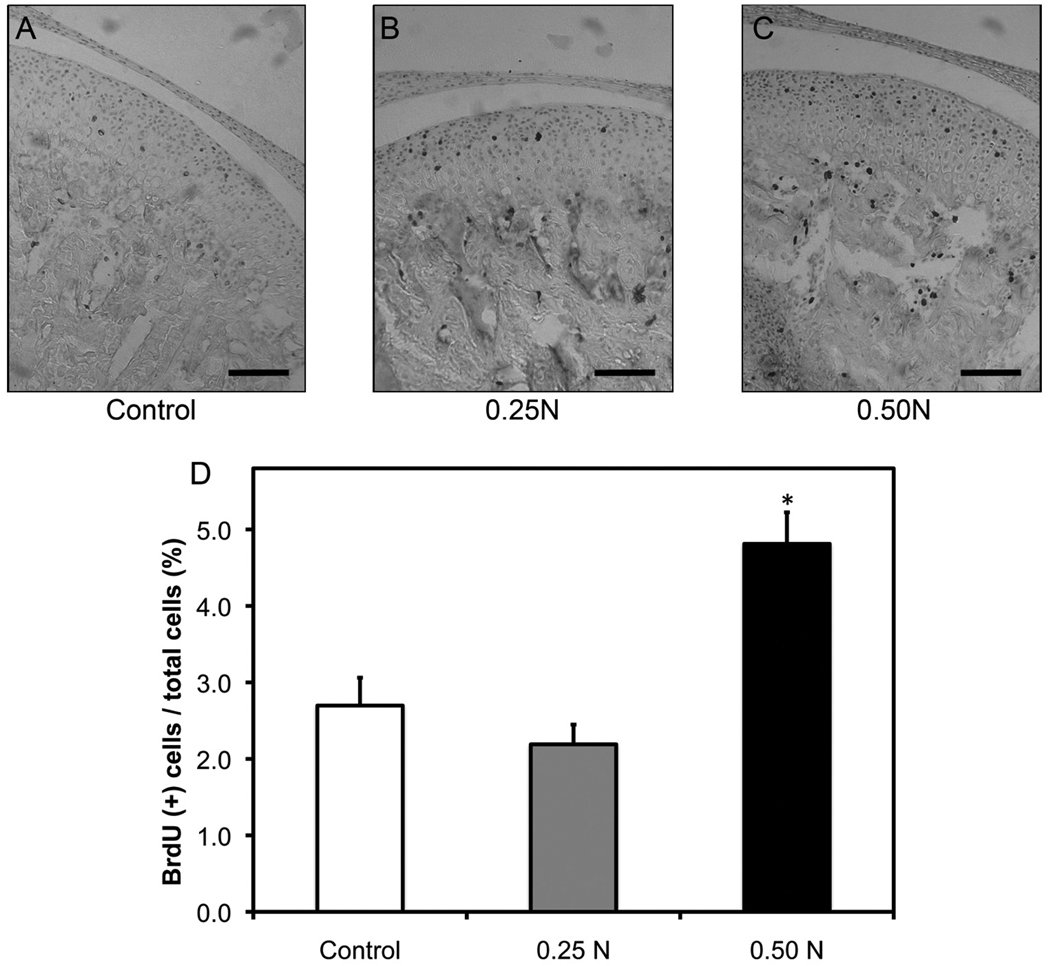
To examine forced mouth opening induced proliferation, we immunostained histological sections of TMJs for BrdU (Figs. 3A, 3B, 3C). After 5 days of forced mouth opening at 0.5 N for 1 hr/day, there was a significant increase in proliferation in the mandibular condylar cartilage (Fig. 3D) compared with that in the nonloaded controls and the 0.25 N of forced mouth opening group.
Figure 3.
Forced mouth opening causes a significantly increased proliferation. Six-week-old female C57BL/6 mice were exposed to normal (A) or forced mouth opening [0.25 N (B) or 0.50 N (C)] for 1 hr/day for 5 days. Points are the means ± SE for n = 8 mice per group. *Significant difference between 0.50 N forced mouth opening and control groups (P < 0.05). Bar: 50 µm.
Forced Mouth Opening Causes an Increase in Subchondral Trabecular Spacing
Micro-CT analysis revealed that forced mouth opening at 0.5 N for 1 hr/day for 5 days caused a significant increase in trabecular spacing and a trend for a significant decrease in bone volume fraction (p = 0.10) compared with normal loading controls (Figs. 4A–4F). Gene expression analysis revealed no significant differences in RANKL, Opg, and Dmp-1 mRNA expression among the groups (Fig. 4G).
DISCUSSION
In this study, we show that 0.5 N of forced mouth opening for 1 hr/day for 5 days caused increased proliferation and expression of chondrocyte maturation markers in the mandibular condylar head, whereas 0.25 N of forced mouth opening caused a trend for increased Sox9 expression. The lack of significance in the 0.25 N forced mouth opening group may be due to the relatively small sample size (n = 8). Studies are planned with a larger sample size (n = 14) to clarify the issue. In other growth plates, static compression can either promote or inhibit growth, depending on the duration of applied loading. Short durations of static compression are anabolic (1 hr/day for 14 days) (Lai et al., 2008), whereas static compression for longer durations (> 6 hrs/day) has been shown to inhibit vertebral growth (Wang et al., 2007; Cancel et al., 2009).
In rodents, fixed functional appliances have also been shown to cause increased proliferation and chondrocyte maturation in the mandibular condylar cartilage (Rabie et al., 2003; Ng et al., 2006; Shen et al., 2006). This is surprising, because forced mouth opening and functional appliances are believed to cause exactly the opposite effects on the positioning of the mandibular condyle. Functional appliance therapy is believed to place the mandible in a protrusive position, causing the condyle to be unseated caudally and ventrally from the articular fossa, while forced mouth opening is believed to cause a compressive force between the articular eminence and the mandibular condyle (Fujisawa et al., 2003). A possible explanation for both functional appliance therapy and forced mouth opening causing similar effects in the mandibular condylar cartilage is that the mandibular condylar cartilage responds similarly to different types of loading compression for forced mouth opening (Kuboki et al., 2000) and tension for functional appliance therapy (Gupta et al., 2009; Panigrahi and Vineeth, 2009). Another possible explanation is that forced mouth opening and functional appliance therapy influence the masticatory muscles in such a way that the actual deformational strains within the mandibular condylar cartilage are similar for both. Strain-gauge measurements of the condylar cartilage have not been made for either modality. It is important to note that making predictions about the loading of the TMJ is difficult and sometimes produces unexpected results. For example, placement of strain gauges in the condylar neck recently demonstrated that, surprisingly, mandibular distraction in minipigs is associated with underloading, not overloading, of the TMJ (Rafferty et al., 2007).
Unlike the mandibular condylar cartilage, in the subchondral bone we found that forced mouth opening caused an increase in trabecular spacing and a trend for decreased bone density. In our decreased occlusal loading model (incisor trimming and soft diet administration), we also found a differential response in the condylar cartilage compared with the subchondral bone (Chen et al., 2009b). Traditionally, static loading in bone is not thought to play a role in mechanotransduction (Forwood and Turner, 1995). However, a recent report has shown that 2N of static loading for 7 min/day for 2 wks caused a significant decrease in trabecular thickness in the tibiae compared with the nonloaded control (Sugiyama et al., 2010), suggesting that short periods of high levels of static loading can suppress bone formation (Robling et al., 2001). We do not know the mechanism behind the decreased subchondral bone density caused by forced mouth opening. Gene expression analysis revealed no significant differences in the expression of the late osteoblast differentiation marker (Dmp-1) or in the expression of osteoclast formation markers (Opg and RANKL). To get a better insight into the mechanism behind the decrease in subchondral bone volume caused by forced mouth opening, future experiments are planned in which we will examine the localization of osteoblast and osteoclast differentiation markers in the subchondral bone and the expression of earlier osteoblast differentiation markers.
Unlike in the rat (Kawai et al., 2008) and rabbit (Fujisawa et al., 2003), we did not find evidence that forced mouth opening caused TMJ osteoarthritis in mice. This may be partially explained by the fact that, in the other rodent studies, investigators used higher levels and longer durations of forced mouth opening forces. We are currently examining whether higher levels of forced mouth opening forces for longer durations in older mice cause TMJ osteoarthritis.
Little is known about the molecular signaling that is involved in mechanical loading induced TMJ remodeling. In this study, we show that forced mouth opening causes increased expression of mandibular chondrocyte maturation markers and a decrease in the subchondral bone volume. Greater understanding of how mandibular condylar cartilage remodeling is controlled by mechanical loading will aid in regenerative therapies for TMJ degenerative diseases and will also help in deciphering the age and gender predilection of TMD.
ACKNOWLEDGMENTS
This work was supported by 5K22DE017193 (SW) and 1R01DE020097 (SW) from the NIH.
Footnotes
REFERENCES
- Benjamin M, Ralphs JR. Biology of fibrocartilage cells. Int Rev Cytol. 2004;233:1–45. doi: 10.1016/S0074-7696(04)33001-9. [DOI] [PubMed] [Google Scholar]
- Cancel M, Grimard G, Thuillard-Crisinel D, Moldovan F, Villemure I. Effects of in vivo static compressive loading on aggrecan and type II and X collagens in the rat growth plate extracellular matrix. Bone. 2009;44:306–315. doi: 10.1016/j.bone.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Chen J, Gupta T, Barasz JA, Kalajzic Z, Yeh WC, Drissi H, et al. Analysis of microarchitectural changes in a mouse temporomandibular joint osteoarthritis model. Arch Oral Biol. 2009a;54:1091–1098. doi: 10.1016/j.archoralbio.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sorensen KP, Gupta T, Kilts T, Young M, Wadhwa S. Altered functional loading causes differential effects in the subchondral bone and condylar cartilage in the temporomandibular joint from young mice. Osteoarthritis Cartilage. 2009b;17:354–361. doi: 10.1016/j.joca.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forwood MR, Turner CH. Skeletal adaptations to mechanical usage: results from tibial loading studies in rats. Bone. 1995;17(4 Suppl):197S–205S. doi: 10.1016/8756-3282(95)00292-l. [DOI] [PubMed] [Google Scholar]
- Fujisawa T, Kuboki T, Kasai T, Sonoyama W, Kojima S, Uehara J, et al. A repetitive, steady mouth opening induced an osteoarthritis-like lesion in the rabbit temporomandibular joint. J Dent Res. 2003;82:731–735. doi: 10.1177/154405910308200914. [DOI] [PubMed] [Google Scholar]
- Gupta A, Kohli VS, Hazarey PV, Kharbanda OP, Gunjal A. Stress distribution in the temporomandibular joint after mandibular protraction: a 3-dimensional finite element method study Part 1. Am J Orthod Dentofacial Orthop. 2009;135:737–748. doi: 10.1016/j.ajodo.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Kawai N, Tanaka E, Langenbach GE, van Wessel T, Sano R, van Eijden TM, et al. Jaw-muscle activity changes after the induction of osteoarthrosis in the temporomandibular joint by mechanical loading. J Orofac Pain. 2008;22:153–162. [PubMed] [Google Scholar]
- Kuboki T, Takenami Y, Maekawa K, Shinoda M, Yamashita A, Clark GT. Bio-mechanical calculation of human TM joint loading with jaw opening. J Oral Rehabil. 2000;27:940–951. doi: 10.1046/j.1365-2842.2000.00582.x. [DOI] [PubMed] [Google Scholar]
- Lai A, Chow DH, Siu SW, Leung SS, Lau EF, Tang FH, et al. Effects of static compression with different loading magnitudes and durations on the intervertebral disc: an in vivo rat-tail study. Spine (Phila PA 1976) 2008;33:2721–2727. doi: 10.1097/BRS.0b013e318180e688. [DOI] [PubMed] [Google Scholar]
- Luder HU, Leblond CP, von der Mark K. Cellular stages in cartilage formation as revealed by morphometry, radioautography and type II collagen immunostaining of the mandibular condyle from weanling rats. Am J Anat. 1988;182:197–214. doi: 10.1002/aja.1001820302. [DOI] [PubMed] [Google Scholar]
- Milam SB. Pathogenesis of degenerative temporomandibular joint arthritides. Odontology. 2005;93:7–15. doi: 10.1007/s10266-005-0056-7. [DOI] [PubMed] [Google Scholar]
- Ng AF, Yang YO, Wong RW, Hägg EU, Rabie AB. Factors regulating condylar cartilage growth under repeated load application. Front Biosci. 2006;11:949–954. doi: 10.2741/1851. [DOI] [PubMed] [Google Scholar]
- Ohno S, Schmid T, Tanne Y, Kamiya T, Honda K, Ohno-Nakahara M, et al. Expression of superficial zone protein in mandibular condyle cartilage. Osteoarthritis Cartilage. 2006;14:807–813. doi: 10.1016/j.joca.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahi P, Vineeth V. Biomechanical effects of fixed functional appliance on craniofacial structures. Angle Orthod. 2009;79:668–675. doi: 10.2319/052708-281.1. [DOI] [PubMed] [Google Scholar]
- Rabie AB, Hägg U. Factors regulating mandibular condylar growth. Am J Orthod Dentofacial Orthop. 2002;122:401–409. doi: 10.1067/mod.2002.125713. [DOI] [PubMed] [Google Scholar]
- Rabie AB, Tang GH, Xiong H, Hägg U. PTHrP regulates chondrocyte maturation in condylar cartilage. J Dent Res. 2003;82:627–631. doi: 10.1177/154405910308200811. [DOI] [PubMed] [Google Scholar]
- Rafferty KL, Sun Z, Egbert M, Bakko DW, Herring SW. Changes in growth and morphology of the condyle following mandibular distraction in minipigs: overloading or underloading? Arch Oral Biol. 2007;52:967–976. doi: 10.1016/j.archoralbio.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robling AG, Duijvelaar KM, Geevers JV, Ohashi N, Turner CH. Modulation of appositional and longitudinal bone growth in the rat ulna by applied static and dynamic force. Bone. 2001;29:105–113. doi: 10.1016/s8756-3282(01)00488-4. [DOI] [PubMed] [Google Scholar]
- Shen G, Darendeliler MA. The adaptive remodeling of condylar cartilage—a transition from chondrogenesis to osteogenesis. J Dent Res. 2005;84:691–699. doi: 10.1177/154405910508400802. [DOI] [PubMed] [Google Scholar]
- Shen G, Rabie AB, Zhao ZH, Kaluarachchi K. Forward deviation of the mandibular condyle enhances endochondral ossification of condylar cartilage indicated by increased expression of type X collagen. Arch Oral Biol. 2006;51:315–324. doi: 10.1016/j.archoralbio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Shibukawa Y, Young B, Wu C, Yamada S, Long F, Pacifici M, et al. Temporomandibular joint formation and condyle growth require Indian hedgehog signaling. Dev Dyn. 2007;236:426–434. doi: 10.1002/dvdy.21036. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Price JS, Lanyon LE. Functional adaptation to mechanical loading in both cortical and cancellous bone is controlled locally and is confined to the loaded bones. Bone. 2010;46:314–321. doi: 10.1016/j.bone.2009.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, Aoyama J, Miyauchi M, Takata T, Hanaoka K, Iwabe T, et al. Vascular endothelial growth factor plays an important autocrine/paracrine role in the progression of osteoarthritis. Histochem Cell Biol. 2005;123:275–281. doi: 10.1007/s00418-005-0773-6. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87:296–307. doi: 10.1177/154405910808700406. [DOI] [PubMed] [Google Scholar]
- Tang GH, Rabie AB, Hägg U. Indian hedgehog: a mechanotransduction mediator in condylar cartilage. J Dent Res. 2004;83:434–438. doi: 10.1177/154405910408300516. [DOI] [PubMed] [Google Scholar]
- Wadhwa S, Kapila S. TMJ disorders: future innovations in diagnostics and therapeutics. J Dent Educ. 2008;72:930–947. [PMC free article] [PubMed] [Google Scholar]
- Wang DL, Jiang SD, Dai LY. Biologic response of the intervertebral disc to static and dynamic compression in vitro. Spine (Phila PA 1976) 2007;32:2521–2528. doi: 10.1097/BRS.0b013e318158cb61. [DOI] [PubMed] [Google Scholar]